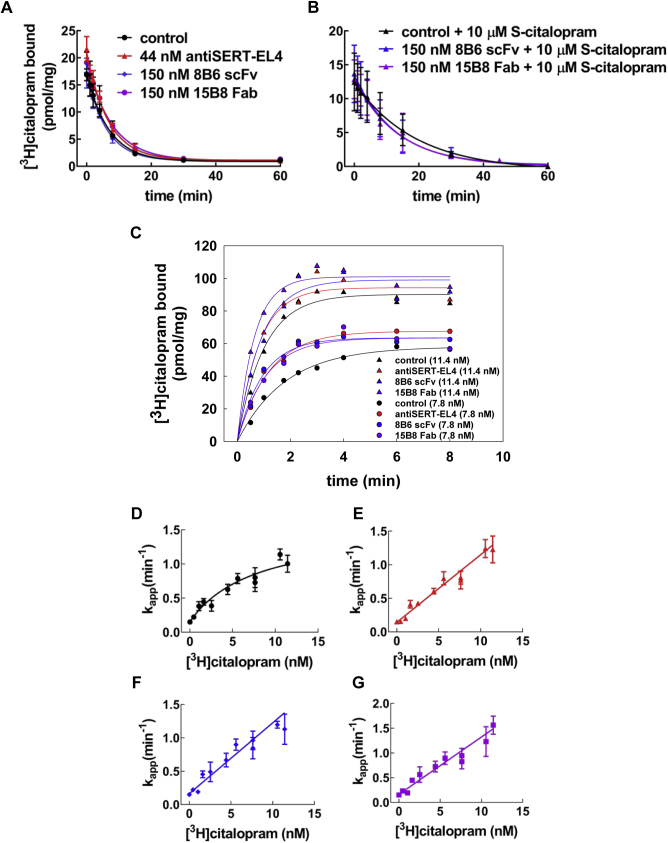
Figure 8.
Kinetics of [3H]citalopram binding to SERT in the presence of 8B6 scFv, 15B8 Fab, or antiSERT-EL4 antibody.A, dissociation experiment: membranes harboring GFP-tagged SERT (1 μg/assay) were incubated with [3H]citalopram (6 nM) in the presence of 8B6 scFv (150 nM), 15B8 Fab (150 nM), or antiSERT-EL4a antibody (44 nM) in a final volume of 10 μl. After 10 min at 25 °C, the dissociation was initiated by 100-fold dilution of the reaction in buffer containing the same concentration of 8B6 scFv, 15B8 Fab, or antiSERT-EL4 antibody. Data are means ± SD from three independent experiments carried out in duplicate. The lines were drawn by fitting the data to the equation for a monoexponential decay. B, dissociation of [3H]citalopram in the presence of 10 μM S-citalopram. The experiment was done as in panel A with 5 nM [3H]citalopram, but 10 μM S-citalopram was present in in the dilution buffer to occupy the S1 site and to thereby slow dissociation of prebound [3H]citalopram. The parallel incubations were done in the absence of S-citalopram. These are not shown for the sake of clarity, but the dissociation rate constants were identical to those determined in panel A. Data are means ± SD from three independent experiments carried out in duplicate. C, association experiment: membranes harboring GFP-tagged SERT (1 μg/assay) were preincubated with 8B6 scFv (150 nM), 15B8 Fab (150 nM), or antiSERT-EL4 antibody (44 nM) for 10 min at 25 °C. Thereafter the binding reaction (final volume 0.1 ml) was initiated by the addition of [3H]citalopram (7.8 nM and 11.4 nM) and terminated by rapid filtration at the indicated time points, bound to membranes prior to the binding reaction. The lines were drawn by fitting the data to the equation for a monoexponential association. Data are means from duplicate determinations in a representative experiment, which was carried out in parallel. D–F, the apparent association rates (kapp ± SE) were obtained from experiments done as shown in panel C and plotted as a function of the [3H]citalopram concentration plots. Under control conditions (D), i.e., in the absence of antibody/Fabs, the resulting plot was better described by the sum of a rectangular hyperbola and a basal term (= koff) than by a straight line (p = 0.02; F-test based on the extra-sum-of-squares principle). In contrast, in the presence of antiSERT-EL4 antibody (E), 8B6 scFv (F), and 15B8 Fab (G), the linear regression (with koff as y-intercept and kon as slope) was an adequate and parsimonious description because there was not any improvement in the fit by assuming a hyperbolic relation.