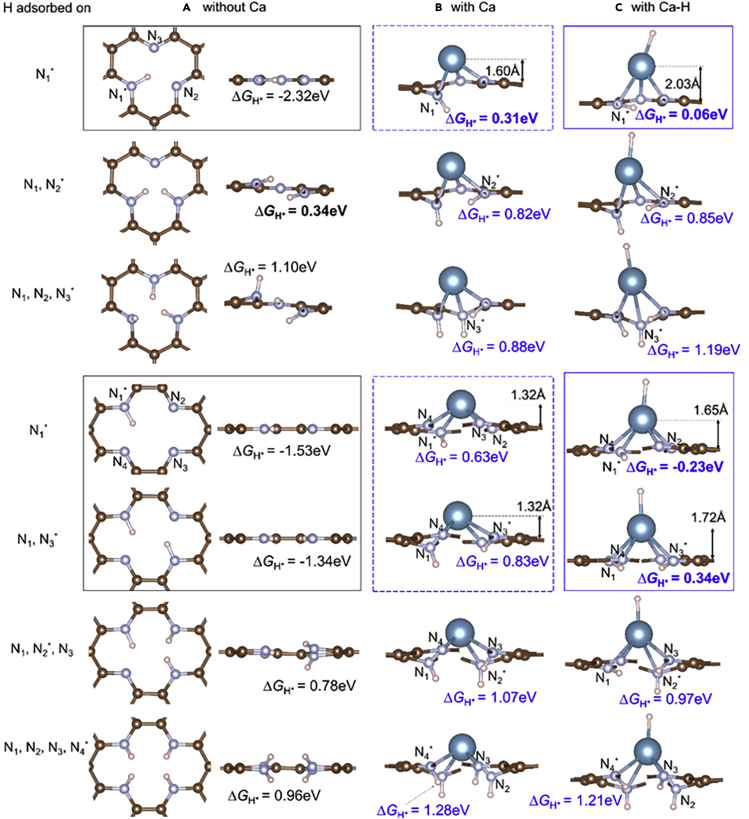
Figure 6.
Atomic structure analysis and hydrogen adsorption
Atomic structures and ΔGH∗ values of H adsorption at each N-site of SV+3N and DV+4N (A) without Ca, (B) with a Ca single atom, and (C) with an extra H adsorbed onto Ca (In the side views some C atoms blocking N and H atoms from our view are not shown). Each ΔGH∗ value is for H adsorption onto the N atom indicated by ∗. When two H atoms are adsorbed onto DV+4N they prefer to adsorb at two N in diagonal positions [N1, N3] rather than neighboring positions [N1, N2], as the diagonal configuration is more stable due to longer separation between the two H∗ (Fujimoto and Saito, 2014). The structures in black boxes are planar, with very strong H binding (very negative ΔGH∗ values). Adsorbing one more H (below black boxes) or depositing a Ca atom (dashed blue boxes) significantly increases ΔGH∗ values to very positive values by changing N-H bond from sp2-like to sp3-like and breaks the charge density interaction between H and other N atoms. Further adsorbing an H onto Ca (blue boxes) reduces the influence of Ca to graphene structure, hence reduces ΔGH∗ to less positive values and makes them more suitable for HER.