Dear Editor,
A recent study in this journal reported that prior SARS-CoV-2 infection is protective even in the absence of a detectable humoral immune response.1 Our prospective longitudinal study was designed to measure the changes in the binding and neutralizing antibody titers in seropositive healthcare workers (HCWs) over a year and the changes in individuals who had and had not been vaccinated. We also assessed the incidence of positive SARS-CoV-2 RNA tests among HCWs who were seropositive for SARS-CoV-2 antibodies and in those who were seronegative.
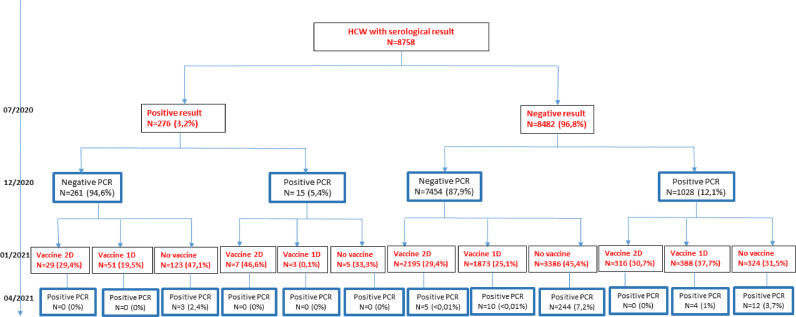
From June 10, 2020 to July 10, 2020, 1 to 2 months after the end of lockdown in France, 8758 HCWs were screened for total serum antibodies by enzyme linked immunosorbent assay (ELISA) kit supplied by Wantai (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, China). All the 276 ELISA-positive personnel identified by the first serological screening were re-tested twice (November 30 to December 9 and March 30 to 15 April) to determine the evolution of SARS-CoV-2 neutralizing antibodies.2 This study was approved by Toulouse University Hospital Ethics Committee (COVIDBIOTOUL RC31/20/0162, CPP number: 20.05.09, CNIL number: 2020-A01292-37).
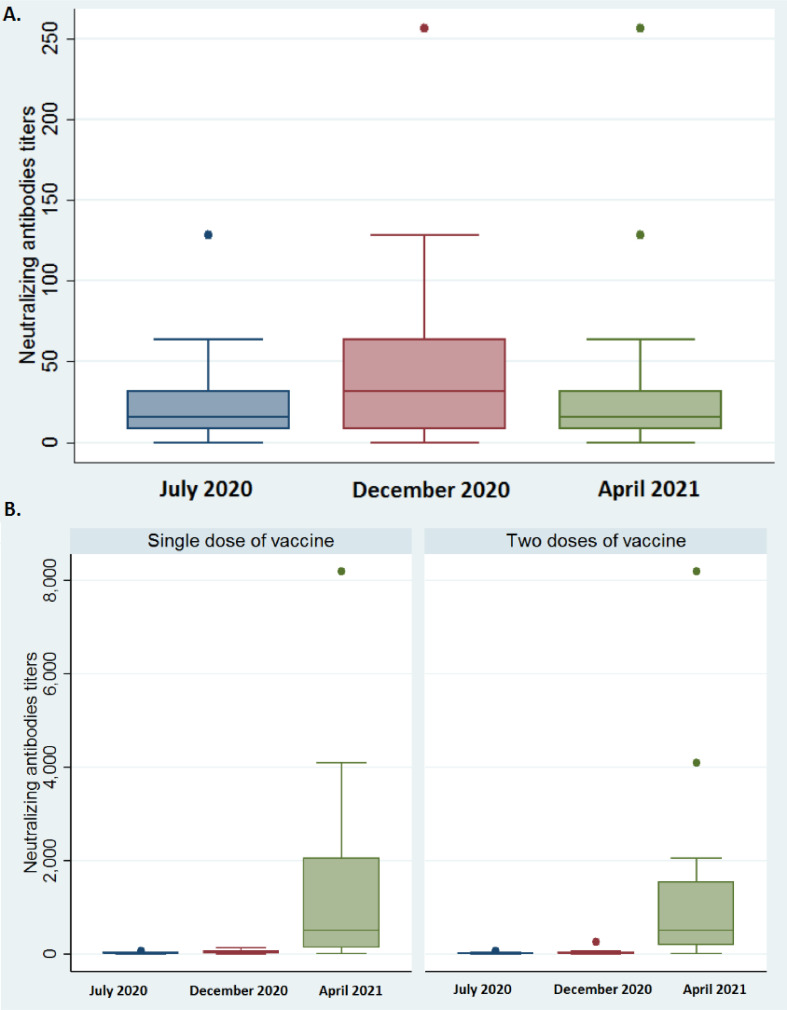
We monitored the ELISA total antibody values and the neutralizing antibody titers of 194 HCWs, 70.3% of the 276 who were serologically positive in July 2020, until April 2021. Only 40 (20.6%) were vaccinated: 17 (42.5%) with two doses of BNT162b2, 16 (40%) with one dose of ChAdOx1 nCoV-19 and 7 (17.5%) with one dose of BNT162b2. The correlation between the Wantai total antibody values and the neutralizing antibodies titers in April 2021 was 76.1% for unvaccinated HCWs and 72.7% for vaccinated HCWs. The December 2020 neutralizing antibody titers (median 32, IQR 8-64) were statistically higher than the April 2021 titers (median 16, IQR 8–32, p = 0.02, Wilcoxon signed rank test, Fig. 1 A). The distributions of neutralizing antibody titers in April 2021 and July 2020 were not statistically different (median 16, IQR [8–32] for both; p = 0.95; Wilcoxon signed rank test, Fig. 1A). The neutralizing antibody titers were significantly higher in April 2021 after both one dose (median 512, IQR [128–2048]) and two doses (median 512, IQR [192–1536]) than in December 2020 (1 dose median 32, IQR [16–64]; p < 0.01, two doses median 16, IQR [8–32]; p < 0.01; Wilcoxon signed rank test, Fig. 1B). The neutralizing antibody titers of HCWs who received one or two doses of vaccine did not differ (p = 0.95, Wilcoxon rank test). All 40 vaccinated HCWs who were infected before July 2020 had higher neutralizing antibody titers in April 2021 than in July 2020. The neutralizing antibody titers for April 2021 were also much higher than those for July 2020 (median 16, IQR [16–32], Fig. 1B), regardless of the number of doses given (p < 0.01; Wilcoxon signed rank tests). The reinfection rate of HCWs first infected before July 2020, median follow-up of 275 days (IQR: 265–281), was 18/276 (6.5%), significantly lower than the first infection rate over the same period (1272/8482; 15%; p < 0.01; Chi2 test) (Fig. 2 ). While 6.5% of unvaccinated HCWs became re-infected within 10 to 13 months of their first SARS-CoV-2 infection, 15% of HCWs who had never been previously infected and were unvaccinated became infected. Thus 56.1% of HCWs were protected against re-infection about one year after their first infection, without being vaccinated.
Fig. 1.
Distributions of neutralizing antibody titers in July 2020, December 2020 and April 2021:
1A: among unvaccinated HCWs
1B: one month post vaccination, among vaccinated HCWs given either one or two doses of vaccine.
Fig. 2.
Study flowchart.
These findings are consistent with the data for neutralizing antibody titers. The distributions in April 2021 and July 2020 were identical: 91.4% of these titers were the same or increased 1 to 3 months after the first infection. However, the neutralizing antibody concentration peaked approximately 9 months after the first infection. The titers for April 2021 were lower than those of December 2020. A previous study on the same sample showed that a high neutralizing antibodies titer protected up to 84.8% HCWs from re-infection for 9 months after their first infection.3 We infer that protection against re-infection peaks at around 9 months after the first SARS-CoV-2 infection, although most of the HCWs remained protected against re-infection a year post-infection, even without vaccination. A recent study on rhesus macaques experimentally infected with SARS-CoV-2 showed that neutralizing antibodies that protected against reinfection developed within 35 days.4 This result, together with those of ourselves and others, indicates that a SARS-CoV-2 infection induces a protective humoral response that can be correlated with the serum neutralizing activity. Further studies are now needed to determine the factors contributing to post-infection protection.
Our results also indicate that vaccination boosts the immune response of infected HCWs. The most striking finding is that there was no re-infection in HCWs vaccinated 9–12 months after their initial SARS-CoV-2 infection. This contrasts favourably with the rates for infected but unvaccinated HCWs. This is undoubtedly due to the very high neutralizing antibody concentrations found in those given one or two doses of vaccines. Maximal protection against SARS-CoV-2 reinfection seems to occur in people who have been infected and vaccinated, although we have no neutralizing antibody titers for HCWs vaccinated but not previously infected. The present results are consistent with those obtained for a small sample; they showed that the neutralizing antibody titers of infected people are lower than those who have been infected and vaccinated.5 However, we found no difference in re-infections or distributions of neutralizing antibodies between HCWs infected before July 2020 who received a single dose of vaccine and those of people with the same infection profile who received two doses. This supports the recent recommendation that people who have already been infected with SARS-CoV-2 should be given a single dose of vaccine,6 even if infection is "old" (over 9 months).
In conclusion, we find that binding and neutralizing antibodies persist for up to one year post-infection. Our data also suggest that vaccinating individuals who have already been infected induces a high level of protection, much higher than that following infection alone. This is particularly important for HCWs, who remain more exposed to SARS-CoV-2 than most of the general population.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all the technicians from the Virology Laboratory who carried out the serologic operations and all the staff who have collected the blood samples for the serologic study.
The English text was edited by Dr Owen Parkes.
Funding
No specific funding.
References
- 1.Breathnach A.S., Duncan C.J.A., Bouzidi K.E., et al. Prior COVID-19 protects against reinfection, even in the absence of detectable antibodies. J Infect. 2021 doi: 10.1016/j.jinf.2021.05.024. May 27S0163-4453(21)00266-8. [DOI] [PubMed] [Google Scholar]
- 2.Dimeglio C., Herin F., Miedougé M., et al. Screening for SARS-CoV-2 antibodies among healthcare workers in a university hospital in southern France. J Infect. 2020 doi: 10.1016/j.jinf.2020.09.035. Sep 30S0163-4453(20)30640-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimeglio C., Herin F., Miedougé M., et al. Protection of healthcare workers against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection. Clin Infect Dis. 2021:ciab069. doi: 10.1093/cid/ciab069. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrashekar A., Liu J., Martinot A.J., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 doi: 10.1126/science.abc4776. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edara V.V., Hudson W.H., Xie X., Ahmed R., Suthar M.S. Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA. 2021;325(18):1896–1898. doi: 10.1001/jama.2021.4388. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise J. Covid-19: people who have had infection might only need one dose of mRNA vaccine. BMJ. 2021;372(308) doi: 10.1136/bmj.n308. Feb 2. [DOI] [PubMed] [Google Scholar]; Houlihan, C.F. et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet Lond. Engl. 396, e6–e7 (2020).