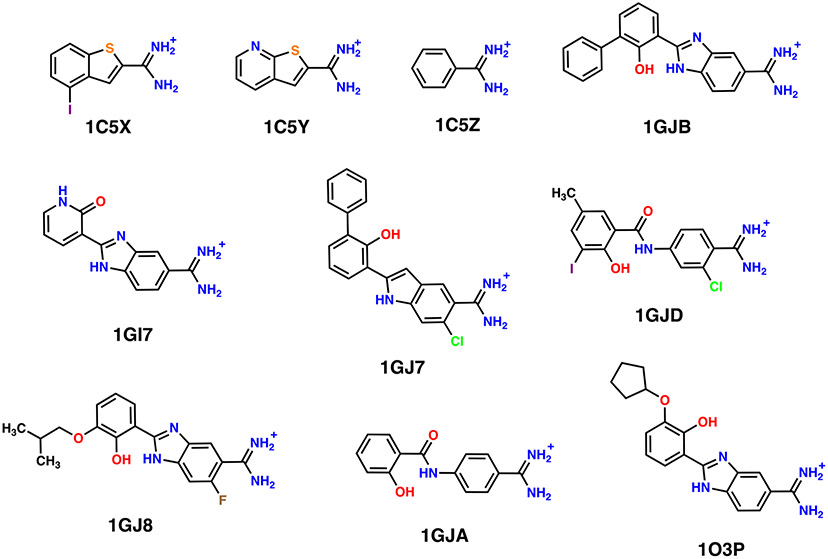
Figure 1.
Chemical structures of the 10 evaluated UPA inhibitors. The molecules share a benzamidine-like scaffold with characteristic amidine group carrying positive charge, and extended tails comprised of a phenol group and other functional modifiers. The hydroxyl on the phenol is proposed to be titratable and samples deprotonated and protonated states during binding, altering the hydrogen bonding capability of the ligands. The inhibitors are categorized as small (those without the phenol group)—1C5X, 1C5Y, 1C5Z, and 1GI7—and big (for those with potentially charged phenols)—1GJ7, 1GJ8, 1GJA, 1GJB, 1GJD, 1O3P.