Abstract
Both genetic and epigenetic information must be transferred from mother to daughter cells during cell division. The mechanisms through which information about chromatin states and epigenetic marks like histone 3 lysine 27 trimethylation (H3K27me3) are transferred have been characterized in animals; these processes are less well understood in plants. Here, based on characterization of a dwarf rice (Oryza sativa) mutant (dwarf-related wd40 protein 1, drw1) deficient for yeast CTF4 (CHROMOSOME TRANSMISSION FIDELITY PROTEIN 4), we discovered that CTF4 orthologs in plants use common cellular machinery yet accomplish divergent functional outcomes. Specifically, drw1 exhibited no flowering-related phenotypes (as in the putatively orthologous Arabidopsis thaliana eol1 mutant), but displayed cell cycle arrest and DNA damage responses. Mechanistically, we demonstrate that DRW1 sustains normal cell cycle progression by modulating the expression of cell cycle inhibitors KIP-RELATED PROTEIN 1 (KRP1) and KRP5, and show that these effects are mediated by DRW1 binding their promoters and increasing H3K27me3 levels. Thus, although CTF4 orthologs ENHANCER OF LHP1 1 (EOL1) in Arabidopsis and DRW1 in rice are both expressed uniquely in dividing cells, commonly interact with several Polycomb complex subunits, and promote H3K27me3 deposition, we now know that their regulatory functions diverged substantially during plant evolution. Moreover, our work experimentally illustrates specific targets of CTF4/EOL1/DRW1, their protein–proteininteraction partners, and their chromatin/epigenetic effects in plants.
Dwarf-related wd40 protein 1 associates with Polycomb complexes to deposit histone 3 lysine 27 trimethylation marks and promotes cell cycle progression in rice.
Introduction
Amino-terminal covalent modifications of histone proteins have a fundamental role in regulating gene expression in multicellular organisms. For example, histone 3 lysine 27 trimethylation (H3K27me3) has been extensively investigated for its function in transcriptional repression (Liu et al., 2010; Pu and Sung, 2015; Schuettengruber et al., 2017). H3K27me3-dependent gene silencing is mediated by two polycomb group (PcG) repressive complexes (PRCs), PRC2 and PRC1, which are conserved in eukaryotes (Mozgova and Hennig, 2015; Förderer et al., 2016). Epigenetic marks are essential in ensuring prompt restoration of proper cell function after cell cycle propagation from mother to daughter cells. During DNA replication at S phase, chromatin structures are fully disrupted and removed from the maternal strand prior to passage of the replication fork and must, therefore, be reassembled on both daughter strands; this reassembly occurs first for H3-H4 marks and then for H2A-H2B dimers (Steffen and Ringrose, 2014). In both animals and plants, PRC2 associates with the replication fork, thereby ensuring stable inheritance of H3K27me3 marks (Petruk et al., 2012; Jiang and Berger, 2017).
The CHROMOSOME TRANSMISSION FIDELITY PROTEIN 4 (CTF4) protein was initially identified in yeast based on its role in chromosome transmission fidelity (Kouprina et al., 1992; Miles and Formosa, 1992). Conserved CTF4 homologs in animals have essential roles in the CELL DIVISION CYCLE 45 (Cdc45)/MINI-CHROMOSOME MAINTENANCE (MCM)/Go, Ichi, Nii, and San (GINS) proteins (known as CMG complex) DNA helicase activity, proper chromatid segregation and cohesion, and error-free DNA damage tolerance in the S phase of the cell cycle (Gambus et al., 2009; Simon et al., 2014; Fumasoni et al., 2015; Sasaki and Kobayashi, 2017). In yeast, CTF4 couples CMG DNA helicase with DNA polymerase α to facilitate parental histone H3-H4 transfer to lagging strands to promote nucleosome assembly (Gan et al., 2018). In Arabidopsis thaliana, the homolog of yeast CTF4 (EOL1) promotes the recruitment of LIKE HETEROCHROMATIN PROTEIN 1 (LHP1) to the LHP1-PRC2 complex to sustain H3K27me3 methylation levels in dividing cells; although disruption of EOL1 does not disrupt flowering, the double eol1 lhp1 mutant has an even shorter flowering time than the lhp1 mutant (Zhou et al., 2017).
Here, we identified a CTF4 putative ortholog in rice (Oryza sativa), dwarf-related wd40 protein 1 (DRW1), which is required for rice development and cell cycle progression. Mutation in DRW1 disrupted normal cell cycle progression. Similar to Arabidopsis EOL1, DRW1 is specifically expressed in actively dividing cells and young tissues, contributes to deposition of H3K27me3 marks, and physically interacts with multiple PcG components (OsCLF, OsLHP1, and OsSWN). Specifically, drw1 exhibited no flowering-related phenotypes (as in the Arabidopsis eol1 mutant), but displayed cell cycle arrest and DNA damage responses. We found that DRW1 sustains normal cell proliferation by modulating the expression of cell cycle inhibitors KIP-RELATED PROTEIN 1 (KRP1) and KRP5, and show that these effects are mediated by the ability of DRW1 to bind their promoters and affect H3K27me3 levels. We also show that DRW1 functions in DNA damage repair: It physically interacts with subunits of a DNA helicase complex and DNA polymerase α, and these interactions are required for DNA replication and to prevent DNA damage. Importantly, no disruption of DNA damage responses was observed in Arabidopsis eol1 mutants. Therefore, our study establishes that CTF4 homologs in different plant species interact with similar regulatory machineries (PcGs), but exert distinct, species-specific functions in highly diverse physiological processes, such as flowering in Arabidopsis thaliana and DNA repair in rice.
Results and discussion
The CTF4-putative-ortholog DRW1 physically interacts with PcG proteins to deposit H3K27me3 modifications
In this study, we identified a mutant drw1 through screening of the Tos17 insertional library of the rice (Oryza sativa spp. japonica) variety “Zhonghua 11” (ZH11) (Wu et al., 2003). Genotyping analysis indicated that the drw1 mutation was caused by a retrotransposon Tos17 insertion on the third exon of the gene LOC_Os09g06560 (Supplemental Figure 1, A–C). BLAST analysis revealed that DRW1 encodes a homolog of yeast CTF4 (Kouprina et al., 1992; Miles and Formosa, 1992) and Arabidopsis EOL1 (Zhou et al., 2017), indicated that this locus is a single-copy gene.
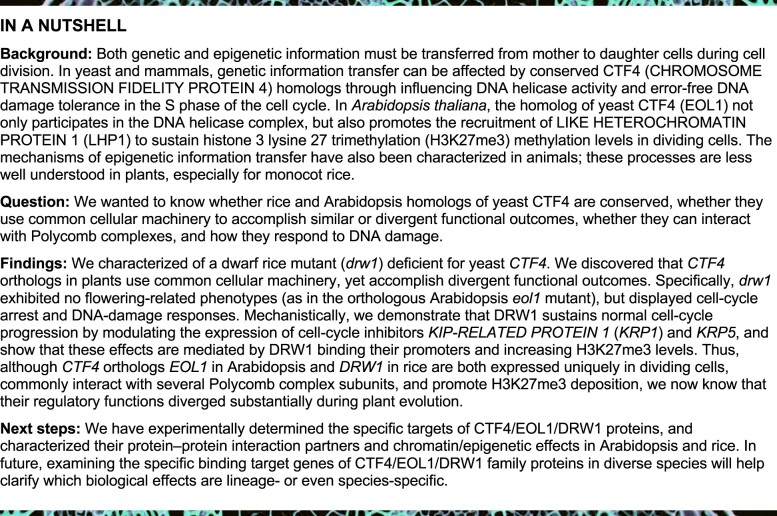
Next, given reports for essential roles of CTF4 in yeast (Gambus et al., 2009; Simon et al., 2014; Fumasoni et al., 2015; Sasaki and Kobayashi, 2017) and Arabidopsis thaliana (Zhou et al., 2017), we examined whether DRW1 exerts conserved functions across species. We first performed sequence alignment and modeling of the 3D structure of DRW1 compared with its yeast homolog CTF4 and Arabidopsis EOL1, and found that CTF4, EOL1, and DRW1 are evolutionarily conserved and each contain a WD40 domain, a six-bladed β-propeller, and a helical domain (Figure 1A). We then transformed rice DRW1 into a yeast double mutant (ctf4-6F16 Δmcm10p) and examined yeast growth at both 25°C and 30°C. We found that rice DRW1 complemented the ctf4-6F16 Δmcm10p growth-defect phenotype (Figure 1B).
Figure 1.
The CTF4-ortholog DRW1 directly interacts with PRC2 and PRC1 components and mediates H3K27me3 in rice. (A) Domain distribution of Saccharomyces cerevisiae CTF4, Arabidopsis thaliana EOL1, and Oryza sativa DRW1 (left). Homology-modeling-based structural prediction of the DRW1 and CTF4 proteins (right); the structures were merged to help identify differences. (B) Yeast complementation assays showing that expression of DRW1 can complement the growth of the ctf4-6F16 Δmcm10-1 double mutant. Ten-fold serial dilutions of log-phase yeast cells (wild-type, ctf4-6F16Δ, ctf4-6F16 Δmcm10-1, mcm10-1, and DRW1+ctf4-6F16 Δmcm10-1) were spotted onto YPD plates and incubated at both 25°C and 30°C for 3–4 days. (C–D) Y2H analysis showing that DRW1 physically associates with OsCLF (C) and OsLHP1 (D) in yeast cells. Full-length DRW1 and OsCLF or OsLHP1 were fused with the GAL4-AD or GAL4-BD domains (as indicated), and were then transformed into yeast cells and grown on SD (–WLHA) selective medium without tryptophan (W), leucine (L), histidine (H), or adenine (A). (E–F) BiFC analysis of the physical interaction of DRW1 with OsCLF (E) and OsLHP1 (F) in rice protoplasts. Yellow fluorescence signals indicate physical associations of interacting partner proteins in nuclei. EYFP fluorescence was observed in rice protoplasts expressing DRW1-cEYFP and nEYFP-OsCLF and in cells expressing DRW1-cEYFP and nEYFP-OsLHP1. Bars represent 20 μm. (G–H) Co-immunoprecipitation assay of DRW1 with OsCLF (G) and OsLHP1 (H) in rice protoplasts. Total protein extracts of protoplasts prepared from transformed double hemizygous DRW1:FLAG and LHP1:HA cells; these were immunoprecipitated with anti-FLAG agarose. Subsequently, the precipitates were analyzed by immunoblotting with an anti-HA antibody to identify OsCLF:HA and OsLHP1:HA. (I) Loss-of-function DRW1 leads to a decrease in H3K27me3 abundance in rice. Histones derived from 9-day-old seedlings of wild-type ZH11 and drw1 mutant in rice, and wild-type Col-0 and eol1 mutant Arabidopsis plants, were analyzed by immunoblotting with antibodies specifically recognizing H3K27me3 modification; the anti-H3 antibody was used as a control. (J) Quantitative analysis of the H3K27me3 level in rice drw1 and Arabidopsis eol1 mutants. Signal intensity was quantified using ImageJ. Data are means ± SD, and n = 3 in (H). Student’s two-tailed t test (**, p < 0.01; n.s., not significant).
Arabidopsis EOL1 functions as an enhancer of LHP1 recruitment and interacts with Polycomb complexes (AtLHP1, AtCLF, and AtSWN) to sustain H3K27me3 methylation levels (Zhou et al., 2017). These clues prompted us to question whether rice DRW1 may have a function related to depositing H3K27me3 through physical interaction with PcG proteins. We first performed yeast two-hybrid (Y2H) assays to identify the interaction partners of DRW1. DRW1 directly interacted with OsCLF (Figure 1C), OsLHP1 (Figure 1D), and OsSWN (Supplemental Figure 2). We also conducted bimolecular fluorescent complementation (BiFC) assays to confirm these interactions in protoplasts prepared from 10-day-old rice seedlings: DRW1 indeed physically associated with OsCLF (Figure 1E) and OsLHP1 (Figure 1F). Subsequent co-immunoprecipitation (Co-IP) assays also successfully verified these interactions in vivo in rice protoplasts (Figure 1, G and H). Together, these results confirmed that DRW1 physically interacts with PcG proteins in rice cells.
In Arabidopsis, AtCLF and AtLHP1 function as subunits of the PRC2 and PRC1 complexes, respectively; these methyltransferase complexes deposit H3K27me3 modifications on silenced chromatin (Turck et al., 2007; Liu et al., 2015). We next examined the global H3K27me3 abundance with immunoblot assays of wild-type ZH11 and drw1 plants and found that the drw1 mutant displayed a significant decrease in overall H3K27me3 abundance compared to wild-type ZH11 (Figure 1, I and J). However, it was highly notable that we detected no difference in overall H3K27me3 abundance when we probed 9-day-old Arabidopsis eol1 and wild-type Col-0 seedlings (Figure 1, I and J). Thus, in light of the fact that both DRW1 and EOL1 can physically interact with Polycomb complexes, the clear epigenetic regulatory and global differences for H3K27me3 abundance between rice drw1 and Arabidopsis eol1 mutants indicate that the CTF4/EOL1/DRW1 proteins have species- and/or cell-type-specific functions, possibly acting as specific targeting adapters to direct the Polycomb machinery in selectively depositing H3K27me3 marks to achieve developmental-stage-specific outcomes that differ among species.
Loss of function of DRW1 in rice caused a severe dwarf phenotype but did not disrupt flowering
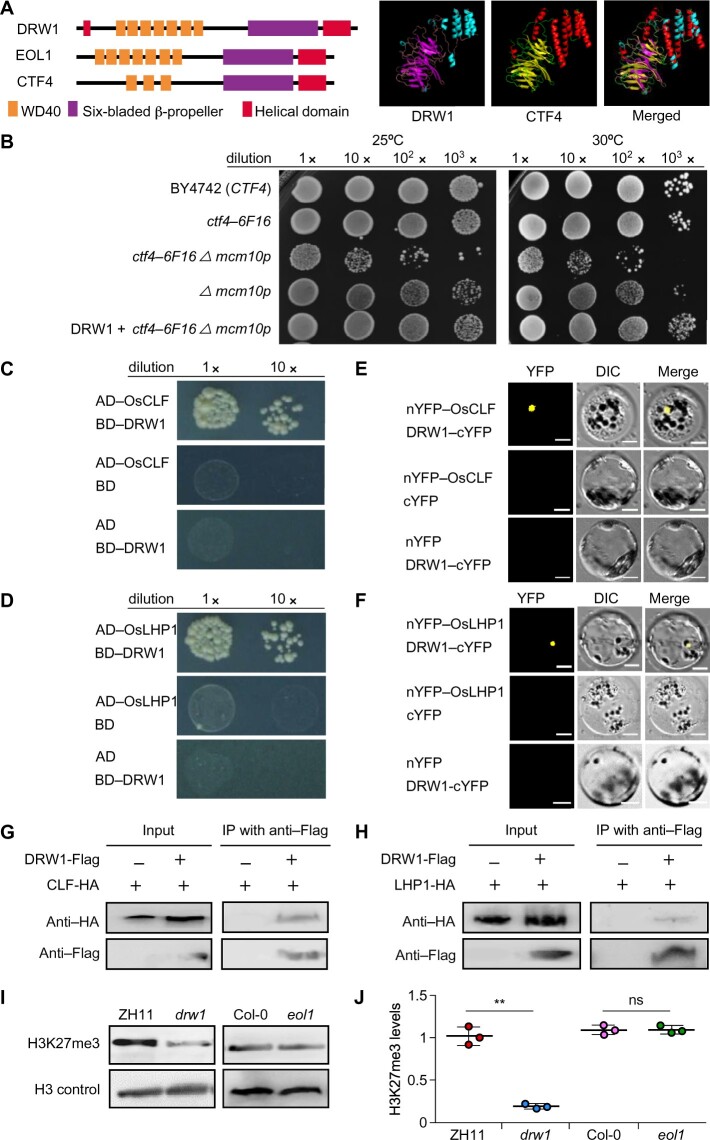
In Arabidopsis, disruption of EOL1 does not disrupt flowering but causes increased expression of flowering-related genes including FLOWERING LOCUS T (FT) and SEPPALATA 3 (SEP3;Zhou et al., 2017). We observed no difference in the flowering time of the rice drw1 mutant (Figure 2, A and B). However, against our expectation, our quantitative reverse transcription-PCR (RT-qPCR)-based analysis of the expression of rice orthologs of known Arabidopsis EOL1 downstream target genes (e.g., SEP3 (OsMADS13), AGAMOUS (OsMADS7), and FT (RFT1 and Hd3a)) revealed no differences between ZH11 and drw1 plants (Supplemental Figure 3A).
Figure 2.
Mutation of DRW1 causes developmental defects and DRW1 is specifically expressed in dividing cells in rice. (A) Phenotypic performance of wild-type ZH11 and drw1 plants. Bar represents 10 cm. (B–C) Bar graphs of heading date (B) and plant height (C). (D–F) Panicle and leaf morphology. Bar represents 10 cm. (G–I) Kernel morphology. Bar represents 10 mm. (J) Subcellular localization of a DRW1-GFP fusion protein in ZH11 rice protoplasts. Blue fluorescence from DAPI (4,6-diamidino-2-phenylindole) staining indicates the nucleus. Bar = 50 μm. (K) Histochemical GUS assays for stably transformed promoter DRW1-GUS fusion lines. GUS signals appear in the shoot apical meristem and in the meristems of primary and lateral roots. Scale bars: SAM, 1 mm; primary root and lateral root, 100 μm. (L–M) RT-qPCR-based analysis of DRW1 transcription in different tissues (L) and in whole plants from 1 week to 6 weeks of age (M). RJ, rhizome junction. Note that the growth stage of panicle tissue was old (it was 21 cm long). Data are means ± SD (standard deviation), n > 15 in (B–C), (E–F), and (H–I), and n = 3 in (L) and (M). Student’s two-tailed t test (*, p < 0.05; **, p < 0.01; n.s., not significant).
We also performed chromatin immunoprecipitation (ChIP) using DRW1-specific polyclonal antibodies in ZH11 and drw1 seedlings. We first isolated total proteins from ZH11 and drw1 plants and performed immunoblotting assays: DRW1 was clearly detected in wild-type plants but not in drw1 mutants (Supplemental Figure 3B). Consistent with the unchanged expression level of flowering time in drw1, we also revealed that DRW1 could not bind at the RFT1 and Hd3a loci and did not affect the H3K27me3 levels at these loci (Supplemental Figure 3, C–F). In addition, the Arabidopsis lhp1 displays an early flowering phenotype that is enhanced in double lhp1 eol1 mutants (i.e. a further shortened flowering time; Zhou et al., 2017); a recent study showed that the rice Oslhp1 mutant functions in extending the vegetative phase (Cui et al., 2020). Thus, although the flowering time of the Arabidopsis eol1 and rice drw1 mutant did not differ from that of wild-type plants, we can speculate that EOL1-LHP1 and DRW1-LHP1 modules may separately regulate downstream biological pathways; for example, DRW1 may not be involved in photoperiodic flowering regulation in rice.
We noted a truly severe dwarf phenotype for the drw1 plants (Figure 2, A and C). Grown under short-day (SD) conditions, we further observed and measured several phenotypes and agronomic traits in wild-type ZH11 and drw1 plants, including sixth leaf length, panicle length, grain size, 1000-grain weight, tiller number, internode length, seed setting rate, cross section of leaf medial vein, and epidermal cell size (Figure 2, D–I; Supplemental Figure 4, A–E). The measured data for all but two of these traits showed significant differences between drw1 and ZH11 plants (only tiller number and epidermal cell size did not differ; Supplemental Figure 4, A and E).
We next constructed a complementation expression vector carrying a ∼6-kb genomic fragment of DRW1 (including about 2.0 kb of the promoter and full-length CDS) and transformed it into the drw1 mutant. The transgenic complementation rescued the dwarf phenotype of drw1: the positive transgenic lines displayed normal plant development and similar plant height as ZH11 (Supplemental Figure 5, A and B). We also performed knockdown and overexpression (OE) genetic experiments to further analyze the function of DRW1. The RNA interference (RNAi) lines generated in the ZH11 background showed a semi-dwarf phenotype compared to the wild-type plants (Supplemental Figure 5, C–E). By contrast, the DRW1 overexpression lines did not differ in height from ZH11 (Supplemental Figure 6, A–C). Taken together, these genetic results establish that DRW1 impacts plant size but not flowering in rice.
DRW1 is localized to the nucleus and is preferentially expressed in dividing cells in rice
In Arabidopsis, EOL1 encodes a nuclear protein expressed in dividing cells (Zhou et al., 2017), so we next probed whether rice DRW1 has a similar subcellular localization and/or expression pattern as Arabidopsis EOL1. For subcellular localization of DRW1, the coding sequence of green fluorescent protein (GFP) was fused to the amino-terminal domain of DRW1 to construct 35S:DRW1-eGFP and we examined transient expression of this fusion construct in ZH11 rice protoplasts prepared from 10-day-old etiolated seedlings. GFP signals from the expressed fusion protein showed that DRW1 is predominantly localized to the nucleus (Figure 2J).
To detect the tissue expression pattern of DRW1 in rice, we further analyzed DRW1 promoter activity in various tissues of pDRW1:GUS transgenic plants. GUS staining revealed that DRW1 is specifically expressed in the rice shoot apical meristem (SAM) and the meristematic zones of lateral and primary roots (Figure 2K). These findings clearly indicate that DRW1 may be specifically expressed in dividing rice cells. Monitoring of DRW1 expression in different tissues of plants grown under SD conditions by RT-qPCR showed that the abundance of DRW1 transcripts was highest in callus and young leaf tissue before heading (Figure 2L). We also found that DRW1 transcript abundance gradually decreased as plants grew (Figure 2M). These results are consistent with findings from Arabidopsis that EOL1 encodes a nuclear protein that is specifically expressed in dividing cells (Zhou et al., 2017).
The drw1 mutant displays a delayed cell cycle S phase, and DRW1 couples the CMG helicase complex to DNA polymerase α
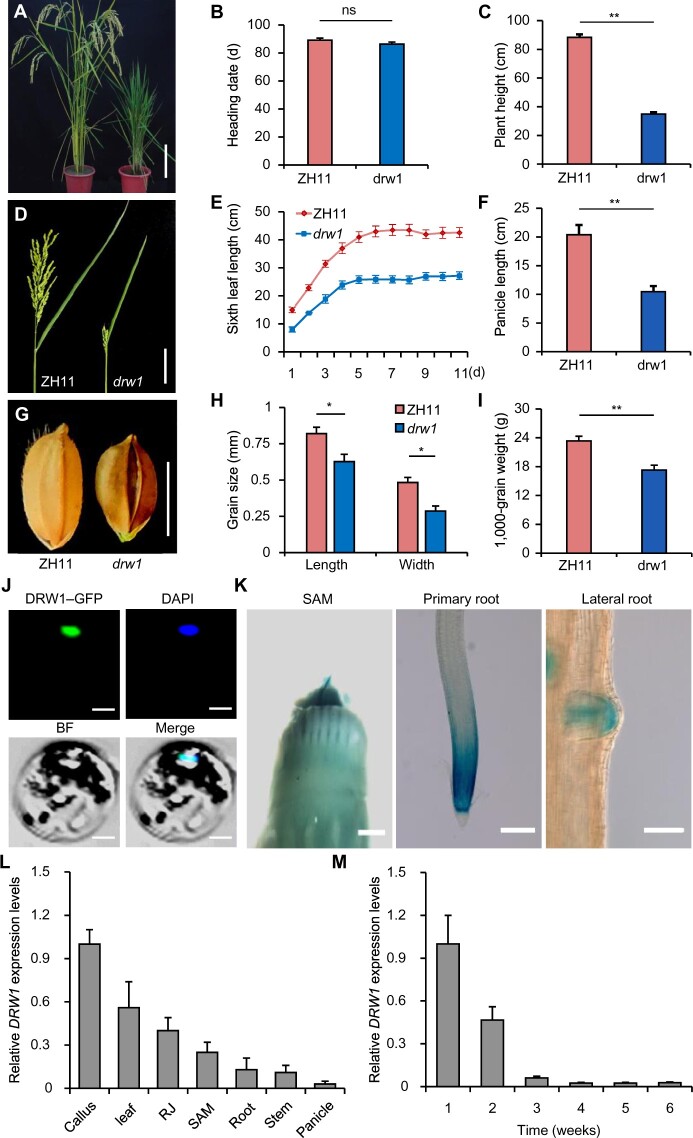
Our above results suggested that nuclear DRW1 may associate with PcG-mediated H3K27me3 modifications in dividing cells. This raised the question of whether DRW1 can confer the cell cycle arrest phenotypes that we initially observed in the dwarf stature drw1 plants. We next performed flow cytometry assays to analyze cell cycle progression in stem apical cells of drw1 and ZH11 plants. The DNA content of drw1 cells at the S stage was significantly higher than that of ZH11 cells, and the drw1 mutant contained significantly more cells at the S phase of the cell cycle (about 31.5%) than ZH11 (14.8%; Figure 3, A and B). Using the EdU (5-ethynyl-2'-deoxyuridine) proliferation assay that was previously used to detect the S phase of the cell cycle in Arabidopsis thaliana and rice (Kotogány et al., 2010; Yoshiyama et al., 2017), we performed EdU labeling on intact roots of germinating rice seedlings of ZH11 and drw1 plants. Germinating rice root tips were incubated for one hour with 100 μM EdU, fixed with formaldehyde in a detergent-containing buffer, and finally washed and incubated with EdU-detection reagent. Similar to our findings from flow cytometry, we found that the drw1 plants had significantly increased numbers of S phase cells (Figure 3, C and D), implying that DRW1 somehow functions in S phase cell cycle regulation in rice.
Figure 3.
Loss-of-function DRW1 delays the cell cycle, and DRW1 regulates cell-cycle-related KRP family genes by promoting H3K27me3 deposition at KRP loci. (A) Cell number during cell cycle phases in ZH11 and drw1 measured by flow cytometry assays. (B) Quantitative analysis of cell number at S stage in ZH11 and drw1 plants. (C–D) Quantitative analysis of EDU treatments on the root meristems of 3- to 5-day-old ZH11 and drw1 seedlings. Bar represents 10 μm. (E–F) DRW1 associates with DNAP (E) and GINS (F) in yeast cells. The full-length DRW1 and DNAP or GINS was fused with the GAL4-AD or BD domain (as indicated), and then transformed into yeast cell grown on SD (–WLHA) selective medium without tryptophan (W), leucine (L), histidine (H), or adenine (A). (G–H) BiFC analysis of the physical interaction of DRW1 with DNAP (G) or GINS (H) in rice protoplasts. Yellow signals indicate physical associations of paired proteins in the nuclei. EYFP fluorescence was observed in rice protoplasts fused with constructs separately encoding nEYFP-DNAP or nEYFP-GINS and DRW1-cEYFP. Bar represents 20 μm. (I–J) RT-qPCR detection of expression of KRP family transcripts in rice (I) and Arabidopsis (J). (K–L) ChIP analysis of DRW1 at the OsKRP5 (K) and OsKRP1 (L) loci. Diagram of the OsKRP5 or OsKRP1 loci (below); the transcribed regions are represented by black boxes, and untranslated regions are represented by gray boxes. Positions of the TSS and the binding regions for the primers used in the ChIP experiments are indicated. (M–N) ChIP analysis of H3K27me3 on the OsKRP5 (M) and OsKRP1 (N) loci. Immunoprecipitated genomic fragments were quantified by qPCR. Data are means ± SD, and n = 3 in (B), (D), and (I–N). Student’s two-tailed t test (**, p < 0.01).
Yeast CTF4 couples with the CMG helicase complex and DNA polymerase α to enable normal DNA replication (Gambus et al., 2009); CTF4 also suppresses the formation of DNA double-strand breaks (Sasaki and Kobayashi, 2017). We used Y2H assays to examine whether DRW1 can physically interact with the rice CMG helicase component OsGINS and/or the rice DNA polymerase α protein OsDNAP. Indeed, DRW1 directly interacted with both proteins (Figure 3, E and F). Further confirming this, in planta BiFC assays with rice protoplasts also showed that DRW1 physically associated with OsGINS and with OsDNAP (Figure 3, G and H). These findings raise the possibility that rice DRW1 may somehow function as a cofactor for DNA replisome function at the S phase of the cell cycle, similar to the known role of CTF4 in yeast.
Interaction of DRW1 with PcGs at OsKRP loci is required for normal cell cycle progression
Given that the monocot rice drw1 mutant displays a marked decrease in the overall H3K27me3 level, and considering the distinct drw1 phenotypes compared to the eudicot Arabidopsis eol1 mutant (Zhou et al., 2017), it is likely that global patterns of gene expression differ dramatically between eol1 and drw1 mutants. An RNA-seq analysis of ZH11 and drw1 seedlings using a 2-fold cutoff with a 5% false discovery rate identified 4,589 differentially expressed genes (DEGs) in rice drw1 compared to the ZH11 plants (Supplemental Figure 7A; Supplemental Data Set 1). Our results above revealed the interactions of DRW1-OsLHP1 and DRW1-OsCLF (Figure 1, C–H). We next examined whether these complex components could mutually affect transcription levels. We did not detect differential transcription levels of OsLHP1 and OsCLF between ZH11 and drw1 plants (Supplemental Data Set 1). Moreover, a recent study did not detect a change in the DRW1 mRNA level in the lhp1 mutant compared to Nipponbare plants (Cui et al., 2020). Further, comparison with DEGs from Arabidopsis eol1 indicated that very few DEGs are shared between rice drw1 and Arabidopsis eol1 (only 30 overlapped DEGs; Supplemental Figure 7B), likely implying that rice DRW1 is involved in diverse biological pathways compared to Arabidopsis EOL1. Guided by previous analyses of cell cycle regulators in plants (Menges et al., 2005; Pettkó-Szandtner et al., 2015), we identified 24 cell-cycle-related genes differentially expressed in drw1 seedlings (Supplemental Figure 7C). In addition, GO analysis of the DEGs between rice ZH11 and drw1 revealed strong enrichment for functional annotations related to cell growth and the cell cycle, findings distinct from the plant-microbiome response predicted pathways among the Arabidopsis DEGs (Supplemental Figure 7D).
Previous reports have revealed that Kip-related proteins (KRPs) inhibit cell cycle progression (Barrôco et al., 2006; Boruc et al., 2010; Mizutani et al., 2010; Coelho et al., 2017). We next performed RT-qPCR to detect expression of KRP family genes in rice. Among the seven OsKRPs, OsKRP1 and OsKRP5 exhibited significantly increased expression in drw1 plants; the drw1 mutant levels of transcripts for the other five did not differ from wild-type ZH11 (Figure 3I). Examination of Arabidopsis KRPs in 9-day-old seedlings revealed, as expected, no significant differences between eo11 and Col-0 plants (Figure 3J). These results support the notion that rice DRW1 but not Arabidopsis EO11 can promote transcription from some KRP loci.
We then performed ChIP-qPCR assays using DRW1-specific polyclonal antibodies in ZH11 and drw1 seedlings. ChIP-qPCR analysis showed that DRW1 was specifically recruited to the 5′ flank regions of the OsKRP5 (Figure 3K) and OsKRP1 (Figure 3M) loci. Moreover, ChIP-qPCR analysis using an H3K27me3-specific antibody in ZH11 and drw1 seedlings showed that the H3K27me3 abundance at the same 5′ flank DRW1-binding regions of the OsKRP5 and OsKRP1 loci was significantly decreased in drw1 seedlings (Figure 3, L and N). In addition, as OsKRP5 is highly expressed in drw1 mutants, we performed ChIP-qPCR assays in wild-type ZH11 and drw1 rice protoplasts to determine whether loss-of-function DRW1 affects OsLHP1 binding at the OsKRP5 promoter. OsLHP1 occupancy at the KRP5 locus was significantly decreased in the drw1 mutant (Supplemental Figure 8), suggesting that DRW1 promotes the recruitment of PRC1 component OsLHP1 to the OsKRP5 locus.
Considering these results together, it appears that the DRW1 protein and H3K27me3 modifications can co-occupy the promoters of two OsKRP genes that function to inhibit cell cycle progression. Further, DRW1 binding at these sites in two OsKRP loci promotes the deposition of H3K27me3 modifications in a manner that ultimately promotes cell cycle progression by repressing the transcription of the two OsKRP genes.
DRW1 functions in DNA damage responses in rice
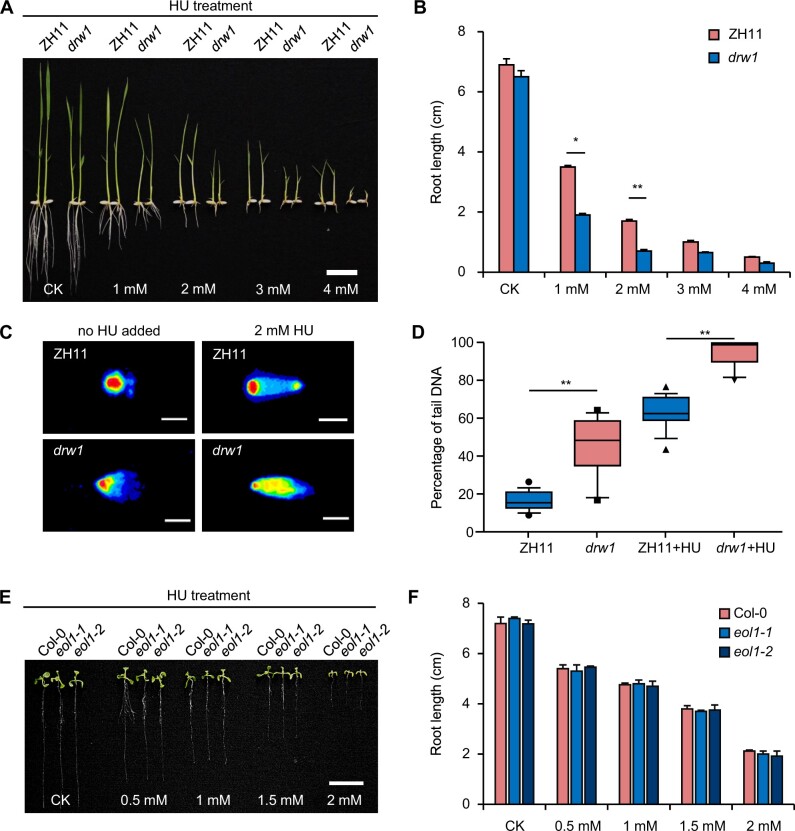
In yeast, CTF4 acts as a helicase-binding protein linking the CMG helicase complex to DNA polymerase α (Gambus et al., 2009; Kang et al., 2013) and is known to be essential for DNA replication and DNA damage repair at the S phase (Gambus et al., 2009; Simon et al., 2014; Fumasoni et al., 2015; Sasaki and Kobayashi, 2017). We therefore checked whether DRW1 could exert similar DNA-damage-related functions in rice. DNA rupture chemical assays based on a range of hydroxyurea (HU) treatment concentrations in wild-type ZH11 and drw1 seedlings indicated that HU treatment (2 mM) caused a significant reduction in the root length of drw1 plants compared to ZH11 (Figure 4, A and B). Similarly, we confirmed that the drw1 root length was also decreased by treatment with another DNA damage reagent, methyl methanesulfonate (MMS; Supplemental Figure 9, A and B).
Figure 4.
DRW1 functions in the DNA damage responses in rice. (A–B) Root morphological characteristics (A) and quantitative analysis of root length (B) of 9-day-old rice seedlings under the indicated HU treatment concentrations. Bar = 2 cm. (C–D) Comet assay (C) and quantitative analysis (D) of 9-day-old ZH11, drw1, ZH11+HU, and drw1+HU seedlings. Blue and red represent DNA strand breaks and complete DNA strand in (C). Bar represents 200 µm in (C). The borders of boxes represent upper and lower quartiles, horizontal lines represent minimum and maximum, and whiskers represent outlier in (D). (E–F) Root morphological characteristics (E) and quantitative analysis of root length (F) of Col and eol1 plants under the indicated HU treatment concentrations. Bar = 2 cm. Data are means ± SD, and n > 25 in (B) and (F), and n > 15 in (D). Student’s two-tailed t test (*, p < 0.05; **, p < 0.01).
We also conducted comet assays to detect whether drw1 mutant rice plants exhibit obvious DNA strand breaks (DSBs): Indeed, the comet tail DNA signal was substantially stronger in drw1 plants, indicating that loss of DRW1 function disrupts the DNA replication capacity of cells exposed to DNA damage (Figure 4, C and D). In agreement with a previous study reporting no difference in eol1 mutants treated by other DNA damaging agents (Zhou et al., 2017), we found that both HU and MMS treatments of Arabidopsis thaliana seedlings caused no difference in the root length (Figure 4, E and F; Supplemental Figure 9, C and D), nor was there any difference in the extent of DSBs between eol1 mutant and Col plants (Supplemental Figure 10, A and B).
Maintenance of DNA integrity is clearly important for inheritance, especially during cell cycle progression—when the plant genome is prone to DNA replication errors and DNA damage (Hu et al., 2016). We interpreted the data about DNA damage to suggest that disruption of the DNA replication process causes a decrease in DNA integrity in drw1 mutants. In drw1, the DNA replication machinery displays arrest: the lack of DRW1 prevents proper coupling of a CMG helicase component (GINS) with DNA polymerase α (DNAP), which can help explain our observation of delayed cell cycle S phase. Together, these results further illustrate divergent, species-specific functional effects for the conserved DRW1/EOL1 proteins.
Materials and methods
Plant materials and growth conditions
The rice (Oryza sativa) mutant drw1 was obtained by screening the T-DNA insertional mutant library in the “Zhonghua” (ZH11; Japonica group) background (Wu et al., 2003). The drw1 mutant, DRW1 complementary lines, DRW1 knockdown lines, and DRW1 overexpression lines were grown under short-day (SD) conditions (28°C, 8 h light/16 h dark) with cool white fluorescent lighting (800 μmol.m−2.s−1) in growth cabinets or under natural SD conditions. The eol1 mutants in the Arabidopsis Col-0 background have been described previously (Zhou et al., 2017). Col-0 and eol1 were grown under long-day (LD) conditions (22°C, 16 h light/8 h dark) with cool white fluorescent light (110 μmol.m−2.s−1). Leaf segments of the sixth leaf from ZH11 and drw1 were embedded in 3% agar until solidification, and then 30-µm transverse sections were prepared using a vibratome (Leica VT 1000 S). Images of the medial veins under ultraluminescence were taken using a microscope (Leica DM 6000M). The primers used in these analyses are detailed in Supplemental Data Set 2.
Flow cytometry
Flow cytometry was conducted with a flow cytometer (Cyflow Space, Partec), following the manufacturer’s instructions, for leaves of ZH11 and drw1 seedlings. Briefly, 2-day-old leaf samples were finely chopped into pieces and incubated in 1 mL of extraction buffer (50 μg/mL PI in 0.1% sodium citrate, 0.05% NP-40), followed by filtering of the homogenate through a Partec 50-µm Cell Trics disposable filter. The filtered nuclei were then subjected to staining with orcein dye, followed by observation with laser excitation at 357 nm.
EDU (5-ethynyl-2'-deoxyuridine) staining
Three-day-old ZH11 and drw1 seedlings were measured for entry into S-phase using a Click-iT EdU Alexa Fluor 488 imaging kit (Invitrogen) according to the manufacturer’s instructions. Briefly, seedlings were submerged in 100 µM EDU for 60 min. After incubation, root tips were fixed with detergent-containing fixer (4% (w/v) formaldehyde solution in phosphate-buffered saline (PBS) with 0.1% Triton X-100) for 30 min at RT and then washed three times with PBS buffer for 10 min. Then, root tips were incubated in EdU detection cocktail (Invitrogen, Click-iT EdU Alexa Fluor 488 HCS assay, Cat#: A10027) for 30 min, followed by PBS-DAPI washes (2 × 5 min washes with PBS containing 100 ng/ml DAPI). The EdU-stained root tips were observed by confocal laser scanning microscopy using a TCSSP8 microscope (Leica).
Β-Glucuronidase (GUS) staining and subcellular localization
The GUS assay was performed as previously reported (Sun et al., 2016). Briefly, the pro-gDRW1-GUS transgenic tissues were incubated in GUS staining solution at 37°C for 8–12 h and then washed with 70% ethanol. The GUS-stained tissues were further imaged using a zoom stereo microscope (Nikon SMZ1000). A vector containing 35S-GFP-DRW1 was transiently expressed in rice protoplasts as described previously (Geng et al., 2020). GFP fluorescence signals were observed and recorded using a Zeiss LSM 700 confocal laser scanning microscope. The primers used in these analyses are detailed in Supplemental Data Set 2.
Yeast complementation assay
We established ctf4-6F16 Δmcm10p double mutant cells in the mcm10p Saccharomyces cerevisiae line (stain BY4742; Ricke and Bielinsky, 2004), according to previously described methods (Amberg et al., 2005). The rice DRW1 gene was cloned into the yeast expression vector pCAMBIA1302, and recombinants were transformed into this double mutant background using an electric shock method. The transformed yeast cell suspensions were serially diluted (1:10) with four gradients and then spotted onto plates for 3- to 5-d incubation at both 25°C and 30°C before analysis. The positive transformants were identified by PCR analysis from a colony DNA template; primers used in these analyses are detailed in Supplemental Data Set 2.
Yeast two-hybrid (Y2H) assays
Constructs for Y2H assays were generated using the Matchmaker Gold Yeast Two-Hybrid System (Clontech) vectors pGBKT7 and pGADT7. The full-length coding sequences (CDS) of DRW1, OsCLF, OsLHP1, OsSWN, OsGINS, and OsDNAP were cloned into the pGADT7 and pGBKT7 vectors and subsequently introduced into the yeast strain AH109, and interactions were assessed by monitoring growth on SD plates lacking His, Trp, and Leu. The primers used in these analyses are detailed in Supplemental Data Set 2.
Bimolecular fluorescence complementation (BiFC) assays
The full-length CDS for DRW1, OsCLF, OsLHP1, OsGINS, and OsDNAP were fused with the coding sequence for an N-terminal EYFP fragment in the nEYFP-N1/pUGW0 (P35S/N-nEYFP) vector and/or for a C-terminal EYFP fragment in the cEYFP-N1/pUGW0 (P35S/N-cEYFP) vector (Nakagawa et al., 2007). BiFC assays were performed as previously described (Sun et al., 2016). Briefly, 10- to 14-d-old rice seedlings were used to isolate rice protoplasts, which were transformed with the constructs. The subcellular localization of GFP fusion proteins was examined using a confocal laser scanning microscope (LSM 700). The primers used in these analyses are detailed in Supplemental Data Set 2.
Co-immunoprecipitation (Co-IP) assays
The full-length CDS of DRW1, OsCLF, and OsLHP1 were fused with amino-terminal FLAG or HA sequences in a vector for expression under the CaMV 35S promoter. The constructs were co-transformed into rice protoplasts using a standard polyethylene glycol method. The co-immunoprecipitation assays were performed as previously described (Gu et al., 2013; Geng et al., 2020). The protein complexes were immunoprecipitated with an anti-FLAG-M2 affinity gel (Sigma, Cat#: A2220) and probed with anti-HA (Roche, Cat#: 12013819001) and anti-FLAG (Sigma, Cat#: A8592) according to standard procedures (Gu et al., 2013). The primers used in these analyses are detailed in Supplemental Data Set 2.
Histone extraction and immunoblot assays
Immunoblot assays were performed as described previously (Gu et al., 2013; Geng et al., 2020). Histones were extracted from 9-day-old ZH11 and drw1, Col and eol1 seedlings using an EpiQuikTM total histone extraction kit (EpiGentek, Cat#: OP-0006) according to the manufacturer’s protocol, followed by separation of proteins on a 4–20% precast gradient SDS–PAGE gel (Bio-Rad, Cat#: 456-1093). H3 Lys acetylation and methylation status were measured via immunoblotting with anti-H3K27me3 (Millipore, Cat#: 07-449) and anti-H3 (Abcam, Cat#: ab1791) antibodies. Note that anti-H3 immunoblotting was performed as a control.
ChIP-qPCR assays
ChIP (chromatin immunoprecipitation) assays were performed with a modified version of previously published methods (Zhang et al., 2012; Geng et al., 2020). Chromatin was extracted from three-week-old rice seedlings and sonicated to produce 200–750 bp fragments, ChIP was performed using the following rabbit polyclonal antibodies: anti-DRW1 (developed in the present study) and commercial anti-H3K27me3 (Millipore, Cat#: 07-449). To determine whether loss-of-function DRW1 affects LHP1’s binding at the OsKRP5 and OsKRP1 promoters, 35S-GFP-LHP1 was transiently expressed in ZH11 and drw1 rice protoplasts as described previously (Geng et al., 2020), followed by ChIP assays with a polyclonal anti-GFP (Abcam, Cat#: ab290) antibody. The purified DNA was then analyzed by qPCR (Supplemental Data Set 2). All assays were performed at least three times with two biological replicates.
RNA-seq and RT–qPCR assays
Three-week-old ZH11 and drw1 seedlings were collected for total RNA extraction. Total RNA was extracted using an Rneasy Plant Mini kit (Qiagen); contaminating genomic DNA was removed by digestion with recombinant Dnase I (Rnase-free, TAKARA) following the manufacturer’s instructions. Three biological replicates were analyzed in the RNA-sequencing (RNA-seq) and RT-qPCR analyses. Analysis of RNA-seq data followed the previous study (Zhang et al., 2018). The raw reads of RNA-seq were mapped to version 7 of the Michigan State University Rice Genome (Ouyang et al., 2007) by Tophat2 (Trapnell et al., 2009), and differentially expressed genes (DEGs) were calculated by Cufflinks (Trapnell et al., 2012). RT-qPCR was performed using SYBR Green Super mix (TOYOBO) on an Applied Biosystems 7500 Fast Real-Time PCR system. Expression levels were normalized to UBQ as an internal reference gene. The primers used in these analyses are detailed in Supplemental Data Set 2.
DNA damage assays
DNA rupture chemical assays tested a range of hydroxyurea (HU) or methyl methanesulfonate (MMS) treatment concentrations on 9-day-old ZH11, drw1, Col, and eol1 seedlings. DNA damage was detected based on a Comet assay using a Comet Assay kit (Trevigen, Cat#: 4250-050-K). Briefly, 9-day-old seedlings were chopped with a razor blade in 10 ml of enzymatic hydrolysate buffer (1.5% cellulase RS, 0.75% macerozyme, 0.6 M mannitol, 10 mM MES, 3.4 mM CaCl2, 0.1% BSA, 5 mM β-mercaptoethanol, 50 µg/ml carbenicillin). Following incubation and preparation of the samples, slides were stained with a 1:10,000 dilution of SYBR Green I stain (Sigma) and were examined using a Nikon Eclipse E800 microscope. The intensity of DNA in each comet tail was quantitatively assessed using ImageJ software (http://rsb.info.nih.gov/ij). DNA damage for each tissue and genotype was calculated by averaging the values for the proportion of tail DNA present in three individual slides, with scoring of 20 comets per slide.
Statistical analysis
For observations of agronomic traits, measurement of cell-cycle-related experiments, gene-expression analyses (RT-qPCR), and ChIP–qPCR, statistical analyses were conducted in Excel 2016 as detailed in the figure legends (Supplemental Data Set 3).
Conclusions
The results of the present study of rice DRW1, considered alongside findings from a previous study of Arabidopsis EOL1 (Zhou et al., 2017), collectively reveal that plant putative orthologs of the yeast protein CTF4 have related molecular functions as adapters to direct the deposition of H3K27me3 marks during plant cell division (Figure 5). However, we also detected obvious differences in the biological impacts of these proteins in the eudicot Arabidopsis thaliana and the monocot rice. In common, AtEOL1 and DRW1 can both physically interact with the PRC2 components CLF and SWN as well as the PRC1 component LHP1. Yet, the flowering-related functions of AtEOL1 are not evident in rice and, vice versa, the DNA-damage-related functions of DRW1 (and yeast CTF4) are not evident in Arabidopsis thaliana.
Figure 5.
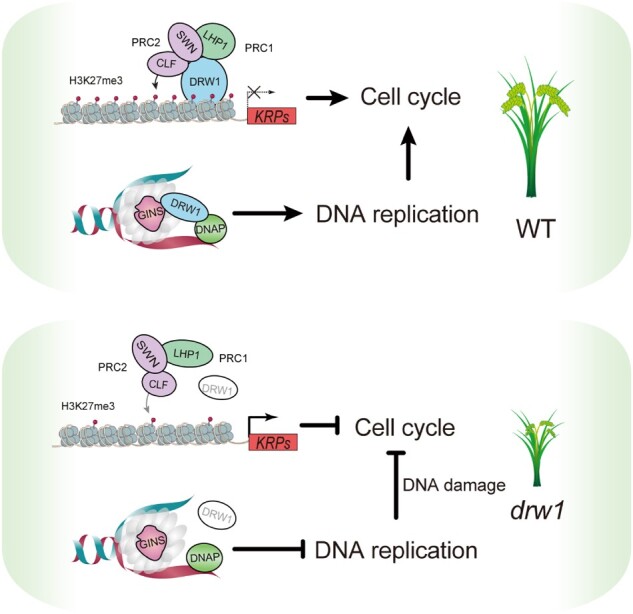
A working model for the molecular functions of DRW1 in rice. DRW1 affects the H3K27me3 modifications through physical interaction with PcGs, including the PRC1 component OsLHP1 and the PRC2 components OsCLF and OsSWN. Similar to yeast CTF4, DRW1 functions in DNA replication by coupling the CMG helicase component (GINS) to DNA polymerase α (DNAP) during cell cycle progression. Lacking DRW1, the disruption of H3K27me3 represses two cell cycle inhibitors OsKRP5 and OsKRP1, which can help explain the delayed cell cycle S phase and severe dwarf phenotype of drw1 plants.
It will be interesting to determine the extent to which the differential functional impacts of plant CTF4 homologs are conserved within various plant lineages. Functional studies in other eudicots and monocots would help clarify this issue. In particular, examination of the specific binding target genes of CTF4/EOL1/DRW1 family proteins in diverse species, as well as characterization of their protein–protein interaction partners, will help clarify which functions represent the general adapter mechanism of these proteins versus which biological impacts are lineage or even species specific. At a minimum, such studies will yield functional insights about basic physiological processes and about any epigenetic regulatory mechanisms that control these processes during cell division in multicellular organisms.
Accession numbers
Sequence data from this article can be downloaded in NCBI (https://www.ncbi.nlm.nih.gov/) or eRice databases (Zhang et al., 2020) under the following accession numbers: DRW1 (LOC_Os09g06560), OsLHP1 (LOC_Os10g17770), OsCLF (LOC_Os06g16390), OsSWN (LOC_Os03g19480), OsGINS (LOC_Os01g14610), OsDNAP (LOC_Os01g64820), OsKRP1 (LOC_Os02g52480), OsKRP5 (LOC_Os09g28580).
Supplemental data
Supplemental Figure 1. PCR-based genotyping of the drw1 mutant.
Supplemental Figure 2. Y2H assay to confirm the interaction of DRW1 with OsSWN.
Supplemental Figure 3. Loss-of-function DRW1 caused no disruption of photoperiodic flowering in rice.
Supplemental Figure 4. Related phenotypes of ZH11 and drw1 mutant plants.
Supplemental Figure 5. Phenotypes of DRW1 complemented and RNAi plants.
Supplemental Figure 6. Phenotypes of DRW1 overexpression plants.
Supplemental Figure 7. Transcriptome-wide analysis of DEGs in rice drw1 and Arabidopsis eol1 mutants.
Supplemental Figure 8. DRW1 promotes the recruitment of PRC1 component OsLHP1 to the OsKRP5 locus.
Supplemental Figure 9. Comparison of DNA damage response between rice drw1 and Arabidopsis eol1 mutants treated with the methyl methanesulfonate (MMS) reagent.
Supplemental Figure 10. Comet assays indicate Arabidopsis eol1 mutants function no response to DNA damage.
Supplemental Data Set 1. List of transcriptome-wide analysis of DEGs between ZH11 and drw1 mutant plants.
Supplemental Data Set 2. List of oligonucleotide primers used in this study.
Supplemental Data Set 3. Detailed statistical analysis in this study.
Supplementary Material
Acknowledgments
This work was supported by the National Transgenic Major Program (2019ZX08010-002) to X.G., the National Natural Science Foundation of China (31630054, 31425018, and 31821005) to C.W., the Key Technologies R & D Program of Tianjin (xya18YFZCNC01210) to S.Y., and the Central Public-Interest Scientific Institution Basal Research Fund (Y2020PT06) to X.G.
Conflict of interest statement. The authors declare no competing interests.
X.G., C.W., and K.Y. designed this research; P.Z., C.Z., and Y.G. performed the main experiments; Y.Y. generated the drw1 mutant; Q.L., A.R., S.C., W.G., L.Y., and S.Y. contributed to data analysis and manuscript writing; and P.Z., Y.G., and X.G. wrote the paper. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is Keke Yi (yikeke@gmail.com) or Changyin Wu (cywu@mail.hzau.edu.cn) or Xiaofeng Gu (guxiaofeng@caas.cn).
References
- Amberg DC, Burker DJ, Strathern JN (2005) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. CSHL Press, New York [Google Scholar]
- Barrôco RM, Peres A, Droual AM, De Veylder L, Nguyen LSL, De Wolf J, Mironov V, Peerbolte R, Beemster GTS, Inzé D, et al. (2006) The cyclin-dependent kinase inhibitor Orysa;KRP1 plays an important role in seed development of rice. Plant Physiol 142: 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruc J, Mylle E, Duda M, de Clercq R, Rombauts S, Geelen D, Hilson P, Inzé D, van Damme D, Russinova E (2010) Systematic localization of the Arabidopsis core cell cycle proteins reveals novel cell division complexes. Plant Physiol 152: 553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho RR, Vieira P, Antonino de Souza Júnior JD, Martin-Jimenez C, De Veylder L, Cazareth J, Engler G, Grossi-de-Sa MF, de Almeida Engler J (2017) Exploiting cell cycle inhibitor genes of the KRP family to control root-knot nematode induced feeding sites in plants. Plant Cell Environ 40: 1174–1188 [DOI] [PubMed] [Google Scholar]
- Cui Y, Cheng J, Ruan S, Qi P, Liu W, Bian W, Ye L, Zhang Y, Hu J, Dong G, Guo L, et al. (2020) The heterochronic gene Oryza sativa LIKE HETEROCHROMATIN PROTEIN 1 modulates miR156b/c/i/e levels. J Integr Plant Biol doi: 10.1111/jipb.12991 [DOI] [PubMed] [Google Scholar]
- Förderer A, Zhou Y, Turck F (2016) The age of multiplexity: Recruitment and interactions of Polycomb complexes in plants. Curr Opin Plant Biol 29: 169–178 [DOI] [PubMed] [Google Scholar]
- Fumasoni M, Zwicky K, Vanoli F, Lopes M, Branzei D (2015) Error-free DNA damage tolerance and sister chromatid proximity during DNA replication rely on the Polα/Primase/Ctf4 complex. Mol Cell 57: 812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus A, Van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, Calzada A, Labib K (2009) A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase α within the eukaryotic replisome. EMBO J 28: 2992–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H, Serra-Cardona A, Hua X, Zhou H, Labib K, Yu C, Zhang Z (2018) The Mcm2-Ctf4-Polα axis facilitates parental histone H3-H4 transfer to lagging strands. Mol Cell 72: 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Zhang P, Liu Q, Wei Z, Riaz A, Chachar S, Gu X (2020) Rice homolog of Sin3-associated polypeptide 30, OsSFL1, mediates histone deacetylation to regulate flowering time during short days. Plant Biotechnol J 18: 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Wang Y, He Y (2013) Photoperiodic regulation of flowering time through periodic histone deacetylation of the florigen gene FT. PLoS Biol 11: e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Cools T, De Veylder L (2016) Mechanisms used by plants to cope with DNA damage. Annu Rev Plant Biol 67: 439–462 [DOI] [PubMed] [Google Scholar]
- Jiang D, , Berger F (2017) DNA replication–coupled histone modification maintains Polycomb gene silencing in plants. Science 357: 1146–1149 [DOI] [PubMed] [Google Scholar]
- Kang YH, Farina A, Bermudez VP, Tappin I, Du F, Galal WC, Hurwitz J (2013) Interaction between human Ctf4 and the Cdc45/Mcm2-7/GINS (CMG) replicative helicase. Proc Natl Acad Sci USA 110: 19760–19765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotogány E, Dudits D, Horváth GV, Ayaydin F (2010) A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouprina N, Kroll E, Bannikov V, Bliskovsky V, Gizatullin R, Kirillov A, Shestopalov B, Zakharyev V, Hieter P, Spencer F (1992) CTF4 (CHL15) mutants exhibit defective DNA metabolism in the yeast Saccharomyces cerevisiae. Mol Cell Biol 12: 5736–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61: 395–420 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou S, Wang W, Ye Y, Zhao Y, Xu Q, Zhou C, Tan F, Cheng S, Zhou DX (2015) Regulation of histone methylation and reprogramming of gene expression in the rice inflorescence meristem. Plant Cell 27: 1428–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, De Jager SM, Gruissem W, Murray JAH (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41: 546–566 [DOI] [PubMed] [Google Scholar]
- Miles J,, Formosa T (1992) Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase alpha, acts in DNA metabolism in vivo. Mol Cell Biol 12: 5724–5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Naganuma T, Tsutsumi KI, Saitoh Y (2010) The syncytium-specific expression of the Orysa;KRP3 CDK inhibitor: Implication of its involvement in the cell cycle control in the rice (Oryza sativa L.) syncytial endosperm. J Exp Bot 61: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgova I,, Hennig L (2015) The polycomb group protein regulatory network. Annu Rev Plant Biol 66: 269–296 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, et al. (2007) The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res 35: D883–D887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk S, Sedkov Y, Johnston DM, Hodgson JW, Black KL, Kovermann SK, Beck S, Canaani E, Brock HW, Mazo A (2012) TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell 150: 922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettkó-Szandtner A, Cserháti M, Barrôco RM, Hariharan S, Dudits D, Beemster GTS (2015) Core cell cycle regulatory genes in rice and their expression profiles across the growth zone of the leaf. J Plant Res 128: 953–974 [DOI] [PubMed] [Google Scholar]
- Pu L,, Sung ZR (2015) PcG and trxG in plants: friends or foes. Trends Genet 31: 252–262 [DOI] [PubMed] [Google Scholar]
- Ricke RM,, Bielinsky AK (2004) Mcm10 regulates the stability and chromatin association of DNA polymerase-α. Mol Cell 16: 173–185 [DOI] [PubMed] [Google Scholar]
- Sasaki M,, Kobayashi T (2017) Ctf4 prevents genome rearrangements by suppressing DNA double-strand break formation and its end resection at arrested replication forks. Mol Cell 66: 533–545 [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G (2017) Genome regulation by polycomb and trithorax: 70 years and counting. Cell 171: 34–57 [DOI] [PubMed] [Google Scholar]
- Simon AC, Zhou JC, Perera RL, van Deursen F, Evrin C, Ivanova ME, Kilkenny ML, Renault L, Kjaer S, Matak-Vinković D, et al. (2014) A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome. Nature 510: 293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen PA, Ringrose L (2014) What are memories made of? How polycomb and trithorax proteins mediate epigenetic memory. Nat Rev Mol Cell Biol 15: 340–356 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang Z, Wu J, Cui X, Feng D, Wang K, Xu M, Zhou L, Han X, Gu X, et al. (2016) The oryza sativa regulator HDR1 associates with the kinase OsK4 to control photoperiodic flowering. PLoS Genet 12: e1005927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V (2007) Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet 3: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Li X, Yuan W, Chen G, Kilian A, Li J, Xu C, Li X, Zhou DX, Wang S, et al. (2003) Development of enhancer trap lines for functional analysis of the rice genome. Plant J 35:418–427 [DOI] [PubMed] [Google Scholar]
- Yoshiyama KO, Kaminoyama K, Sakamoto T, Kimura S (2017) Increased phosphorylation of ser-gln sites on SUPPRESSOR OF GAMMA RESPONSE1 strengthens the DNA damage response in Arabidopsis thaliana. Plant Cell 29: 3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cheng Z, Qin R, Qiu Y, Wang JL, Cui X, Gu L, Zhang X, Guo X, Wang D, et al. (2012) Identification and characterization of an Epi-allele of FIE1 reveals a regulatory linkage between two epigenetic marks in rice. Plant Cell 24: 4407–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wang Y, Chachar S, Tian J, Gu X (2020) eRice: a refined epigenomic platform for japonica and indica rice. Plant Biotechnol J 18: 1642–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liang Z, Cui X, Ji C, Li Y, Zhang P, Liu J, Riaz A, Yao P, Liu M, Wang Y, et al. (2018) N6-methyladenine DNA methylation in Japonica and Indica rice genomes and its association with gene expression, plant development, and stress responses. Mol Plant 11: 1492–1508 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tergemina E, Cui H, Förderer A, Hartwig B, James GV, Schneeberger K, Turck F (2017) Ctf4-related protein recruits LHP1-PRC2 to maintain H3K27me3 levels in dividing cells in Arabidopsis thaliana. Proc Natl Acad Sci USA 114: 4833–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.