Abstract
Background:
Despite continued increases in use of allogeneic hematopoietic cell transplantation (alloHCT) among older adults, no standardized geriatric assessment (GA) has been established to risk-stratify for transplant-related morbidity. We conducted a survey of transplant physicians to determine perceptions of the impact of older age (≥60 years) on alloHCT candidacy, and utilization of tools to gauge candidacy.
Methods:
We conducted a 23-item, online cross-sectional survey of HCT physicians caring for adults in the United States between May and July 2019.
Results:
Of the 770 invited HCT physicians, 175 (22.7%) completed the survey. The majority of respondents were 41–60 years old, male, and practiced in a higher volume teaching hospital. When considering regimen intensity, 29 physicians (17%) stated they would consider a myeloablative regimen for patients ≥70 years, and 141 (82%) would consider reduced intensity/non-myeloablative conditioning for patients ≥70 years. Almost all (90%) endorsed the need for a specialized assessment of pre-HCT vulnerabilities to guide candidacy decisions for older adults. Most physicians reported their centers rarely (33%) or never (46%) utilize a dedicated geriatrician/geriatric-oncologist to assess alloHCT candidates ≥60 years. Common barriers to performing a GA included uncertainty about which tools to use, lack of knowledge and training, and lack of appropriate clinical support staff.
Conclusions:
Many alloHCT physicians will consider alloHCT in patients up to age 75 years and not uncommonly, in patients older than that. However, application of tools and domains varies widely to assess candidacy in older adults. Incorporation of a standardized pre-transplant health assessment tool for risk stratification is a significant unmet need.
Keywords: older adults, allogeneic transplant, geriatric assessment, physician survey
BACKGROUND:
Timely receipt of allogeneic hematopoietic cell transplantation (alloHCT) is a potentially curative therapy for many hematologic diseases. With improvement in donor selection, transplant-specific conditioning regimens, and supportive care, alloHCT is now offered to a wider number of patients, most notably expanding to those with advanced age. In 2018, 39% of alloHCT recipients in the United States (US) were 60 years and older, with 9% of alloHCT performed in recipients age ≥70 years.(1, 2) Reports have demonstrated both feasibility and encouraging outcomes after alloHCT in the older adult population in light of the dismal outcomes of hematologic malignancies with non-transplant approaches.(3)
Underutilization of alloHCT in older adults persists despite these successes (4–7) even for the most common alloHCT indication, acute myeloid leukemia (AML). Bhatt and colleagues reported in patients age 61–75 years with AML that only 5.5% of patients ultimately underwent transplantation.(4) Physician perceptions of eligibility are one of the most important modifiable barriers that could help facilitate early referral to transplant center. The traditional prognostic factors influencing transplant physicians’ estimates of alloHCT-related morbidity and mortality are performance status, comorbidity burden, and chronological age.(8, 9) Specifically, poor performance status and high comorbidity, although not necessarily prohibitive, predict higher non-relapse mortality (NRM) and worse survival.(10, 11)
Use of chronologic age alone to stratify risk poses challenges, especially in the era of adoption of alloHCT for patients into their ninth decade of life.(6) Geriatric assessment (GA), a multi-dimensional health evaluation of older adults, may identify unrecognized impairments and stratify risk for NRM and mortality among older patients.(12–14) The increasing use of alloHCT among older adults has stimulated interest in how best to weigh chronologic age versus patient fitness through GA or other tools prior to alloHCT. Such assessments can also help identify modifiable patient-specific risk factors prior to HCT. Based on an emerging body of data on GA to risk-stratify for morbidity in the general cancer population, national guidelines now recommend GA in patients 65 years or older prior to chemotherapy.(15)
To improve our understanding of transplant physicians’ perceptions when considering candidacy of older adults for alloHCT, we conducted a cross-sectional survey focusing on usual practices in this population. We also explored the utilization of GA and other specialized health testing and barriers to implementation in clinical practice. We hypothesized that chronologic age continues to impact alloHCT candidacy and treatment decisions. We further wanted to describe practice patterns and barriers for health assessment utilizing a GA in the context of alloHCT.
METHODS
This study was a cross-sectional, web-based survey of transplant physicians. Transplant physicians were recruited from the Center for International Blood and Marrow Transplant Research (CIBMTR®) physician mailing list. Given the differences in practice models and patient eligibility criteria across different countries, we focused on US based providers to establish baseline preferences in this demographic. Thus, eligible participants were board certified hematologists or oncologists providing care for adult patients undergoing alloHCT in the US.
Between May and July 2019, a 23-item online survey was sent to 836 potentially eligible adult and adult/pediatric alloHCT physicians (Appendix 1). Non-responders were contacted by four follow-up emails in the weeks following the initial invitation.
Study Measures
Survey questions were developed by members of the protocol team, with input from physician members of the American Society of Transplant and Cellular Therapy (ASTCT) interested in the topic. The 23-item survey was sent via email using web-based survey software, SurveyGizmo (Boulder, CO). Survey domains included 1) physician and transplant center demographics (8 questions, with the first 3 being to establish eligibility to participate), 2) individual and center practice for patients age ≥60 years including consideration of currently utilized functional assessment tools (11 questions), and 3) availability of comprehensive GA and utilization of geriatric specialist program (3 questions). One additional question was asked about interest in future studies (data not reported). When applicable, questions were formulated using a 5-point Likert scale. The survey was piloted by a convenience sample (n=5) to ensure feasibility, interpretability, and novelty, and was revised based on input. Estimated time for completion was approximately10 minutes. Physicians who participated in survey development and the pilot were excluded from participating in the survey itself and were not invited to complete the survey. The National Marrow Donor Program Institutional Review Board classified this study as exempt, determining it was not human subjects research as defined by 45 CFR 46.102(d). All responses were considered anonymous.
Analysis
Survey responses were exported from SurveyGizmo to Excel and imported to SAS Enterprise Guide 9 (Cary, IN) for analysis. Descriptive statistics were computed for all survey questions and demographics. Chi-square or Fisher’s exact test for association was used to examine differences in responses based on participant characteristics. Logistic regression was used to assess for association between demographic factors and whether GA routinely impacted treatment decisions for alloHCT recipients. Variables included in the model were physician age group (30–40, 41–60, ≥61 years), gender, practice setting (teaching hospital-affiliated with university academic center vs. other), years of practice (≤10, 11–20, >20), and center volume of alloHCTs performed on an annual basis (≤49, 50–199, ≥200). A P-value of <0.05 was considered statistically significant.
RESULTS
Participant Characteristics
Of the 836 potentially eligible participants, 770 were eligible, of whom 175 completed more than 75% of the survey questions for an overall survey response rate of 23% (Figure 1). The majority of respondents were 41–60 years old, male, and practiced in a teaching hospital affiliated with an academic center (Table 1). Over 75% reported working at centers performing 50 or more alloHCTs per year. Additionally, a majority of the respondents had more than 10 years of post-training experience in alloHCT, with 43% having more than 20 years.
Figure 1.
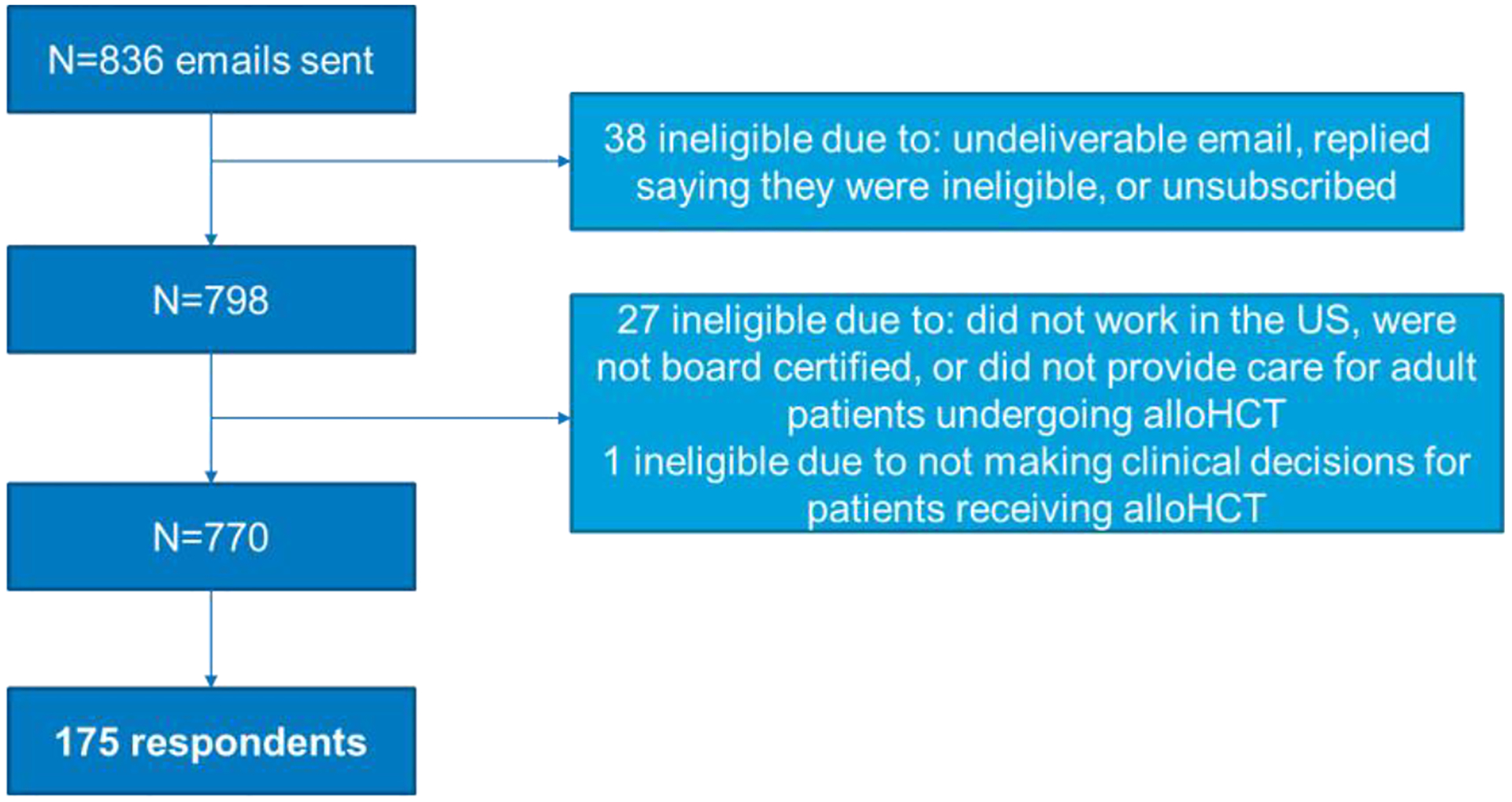
CONSORT Diagram
Table 1.
Respondent Characteristics
| Characteristics | N (%) |
|---|---|
| Total | 175 (100) |
| Age (years) | |
| 30–40 | 33 (18.9) |
| 41–60 | 92 (52.6) |
| ≥61 | 50 (28.6) |
| Gender | |
| Female | 55 (31.4) |
| Male | 119 (68.0) |
| Prefer not to respond | 1 (0.6) |
| Years of post-training experience in alloHCT | |
| ≤10 | 61 (34.9) |
| 11–20 | 38 (21.7) |
| >20 | 76 (43.4) |
| Practice setting | |
| Teaching hospital affiliated with university academic center | 150 (85.7) |
| Other* | 25 (14.3) |
| Annual alloHCT volume by center | |
| <49 | 41 (23.4) |
| 50–199 | 78 (44.6) |
| ≥200 | 56 (32.0) |
Other practice settings by physician report included: teaching hospital not affiliated with university/academic center (n=12); non-teaching hospital (n=10); government hospital- National Institutes of Health clinical center; National Cancer Institute-designated cancer center; and office/clinic not affiliated with a hospital
Note: alloHCTs indicates allogeneic hematopoietic cell transplant
Conditioning Regimen Intensity
Nearly all physicians (91%) reported they would consider alloHCT for patients as old as 75 years, particularly when considering a reduced intensity/non-myeloablative conditioning (RIC/NMA) alloHCT. Detailed responses for considering upper age limit (UAL) of alloHCT by conditioning regimen are shown in Figure 2. The UAL for myeloablative conditioning (MAC) ranged from 50 to 80 years. The majority of respondents (n=139, 79.4%) noted an UAL of age 65 or less, with 16.6% (n=29) stating they would consider MAC for patients ≥70 years, and 7 (4.0%) respondents indicated they did not have an UAL. In contrast, most physicians would consider reduced intensity/non-myeloablative conditioning (RIC/NMA) alloHCT beyond the age of 70 years with over a third of physicians reporting that they would consider such a regimen up to 80 years of age (N=35, 20.0%), or had no UAL (N=31, 17.7%).
Figure 2.
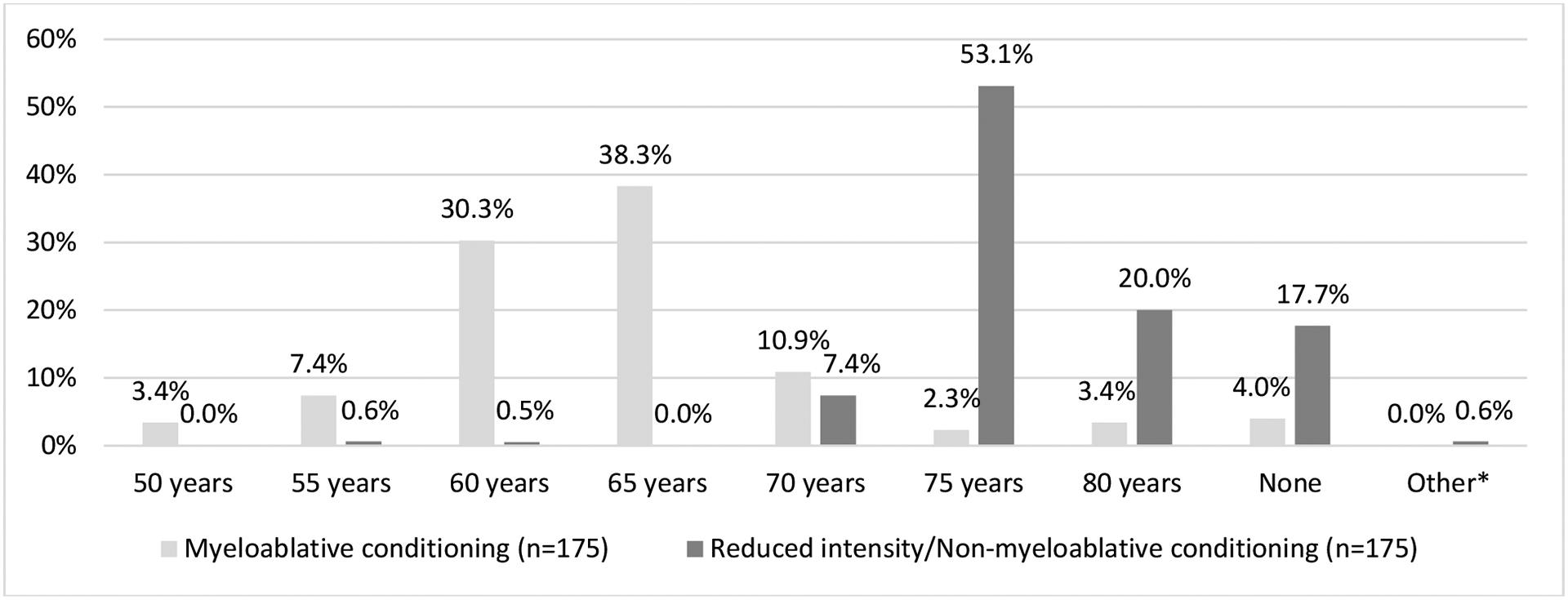
Upper age limits for considering alloHCT for patients using myeloablative or reduced intensity/non-myeloablative conditioning regimens.
*One “other” response included “78” years.
Tools for Evaluating alloHCT Candidacy
Most respondents (n=116, 66%) agreed/strongly agreed that Karnofsky performance score (KPS) is a good reflection of overall function in older adults. The minimum KPS threshold most commonly reported was ≥70 (n=66, 39.1%) or ≥80 (n=72, 42.6%). Twenty respondents (11.4%) used a KPS of ≥90% whereas 6.3% (n=11) had no minimum KPS.
We queried physicians on their usage of recommended GA tools to capture vulnerabilities in older adults.(15) The majority (n=130, 74%) applied measures other than KPS to ascertain functional or health status in such patients prior to alloHCT; 63% (n=110) used other tools routinely while 11% (n=20) prescribed individualized application. Figure 3 summarizes the heterogeneity in physician practice for supplemental tools to ascertain alloHCT candidacy in older patients.
Figure 3.
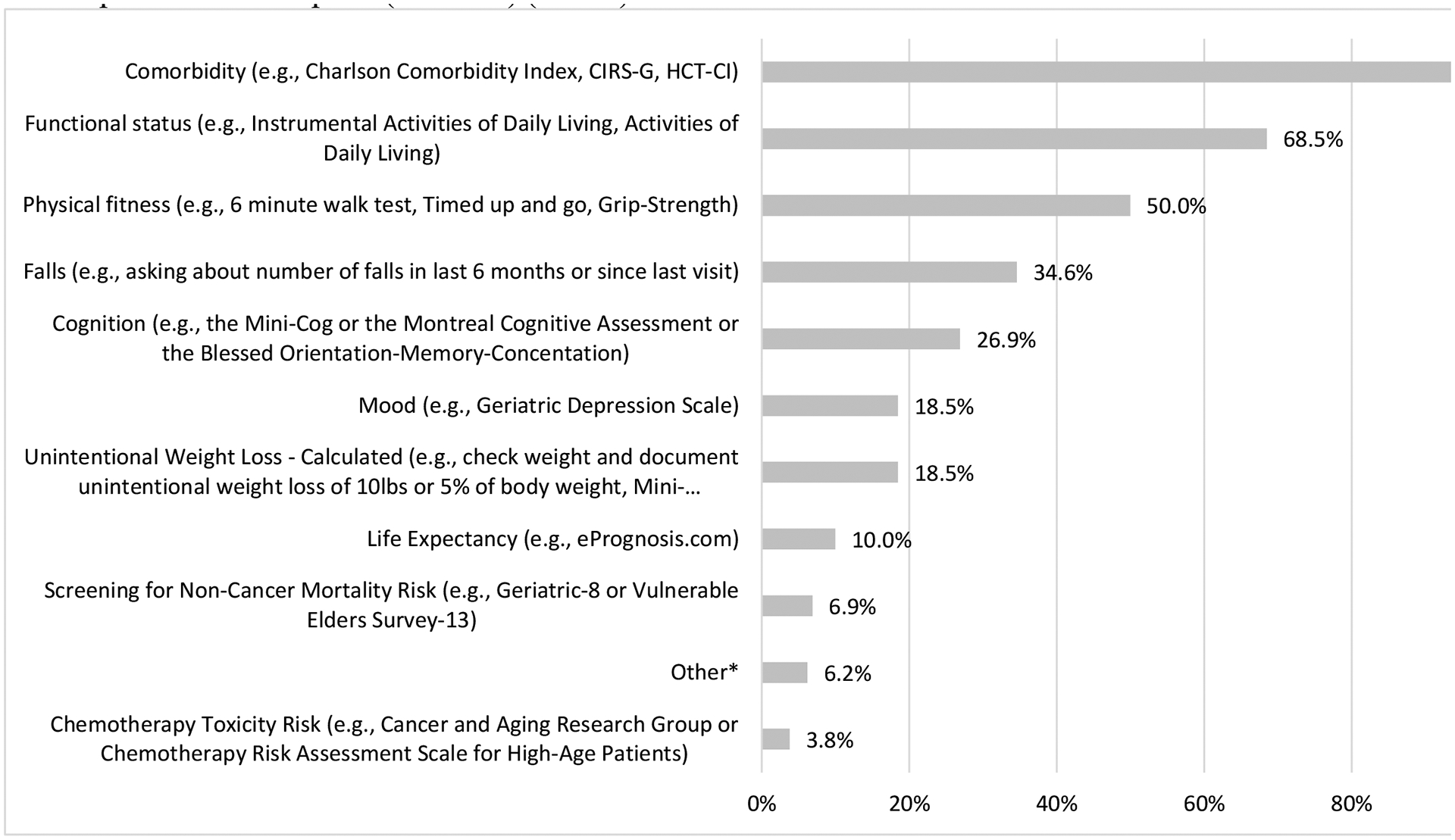
Domains beyond performance status (i.e., Karnofsky performance status) used in the past 12 months to assess patients age 60 years and older being considered for allogeneic hematopoietic cell transplant (alloHCT) (n=130)
*“Other” responses included: “AMPAC [Activity measure for post-acute care] Activity Score”; Activity measure for post-acute care]; “Geriatric assessment”; “PAM Score pretransplant assessment of mortality per [transplant center]”; “Physical therapy assessment”; “social work assessment”; “clinical trial for geriatric population assessment”; “full formal Geriatric assessment”; “our center is just beginning to screen alloHCT candidates that are 60 years of age or older”; “[The] whole patient and his/her support system”
CIRS-G: Cumulative Illness Rating Scale-Geriatric
HCT-CI: Hematopoietic Cell Transplantation-specific Comorbidity Index
Physicians who employed tools other than KPS to characterize functional status were further queried (n=130) to determine their perceptions of whether the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI), the best validated index for NRM after alloHCT, reflected overall health in older adults. Most physicians agreed or strongly agreed (N=86, 66%) that the HCT-CI reflects overall health; the remaining third of physicians did not agree (n=18, 14%) or were neutral (n=26, 20%). Fifty-four percent (N=94) of physicians stated specific HCT-CI scores would exclude a patient from undergoing HCT, as follows: HCT-CI ≥ 2 (n=2, 1%), HCT-CI ≥3 (n=23, 13%), HCT-CI ≥4 (n= 69, 39%). In contrast, 42% (N=74) did not exclude based on an HCT-CI score.
The survey captured physicians insight on the need for specialized assessment of older adults. Nearly all physicians (n=157, 90%) either agreed or strongly agreed that a specialized assessment of pre-transplant vulnerabilities would help determine alloHCT candidacy for older adults. Figure 4 illustrates at which age a standardized toolkit to evaluate alloHCT candidacy was considered most critical in addition to currently utilized measures (such as age, KPS, HCT-CI).
Figure 4.
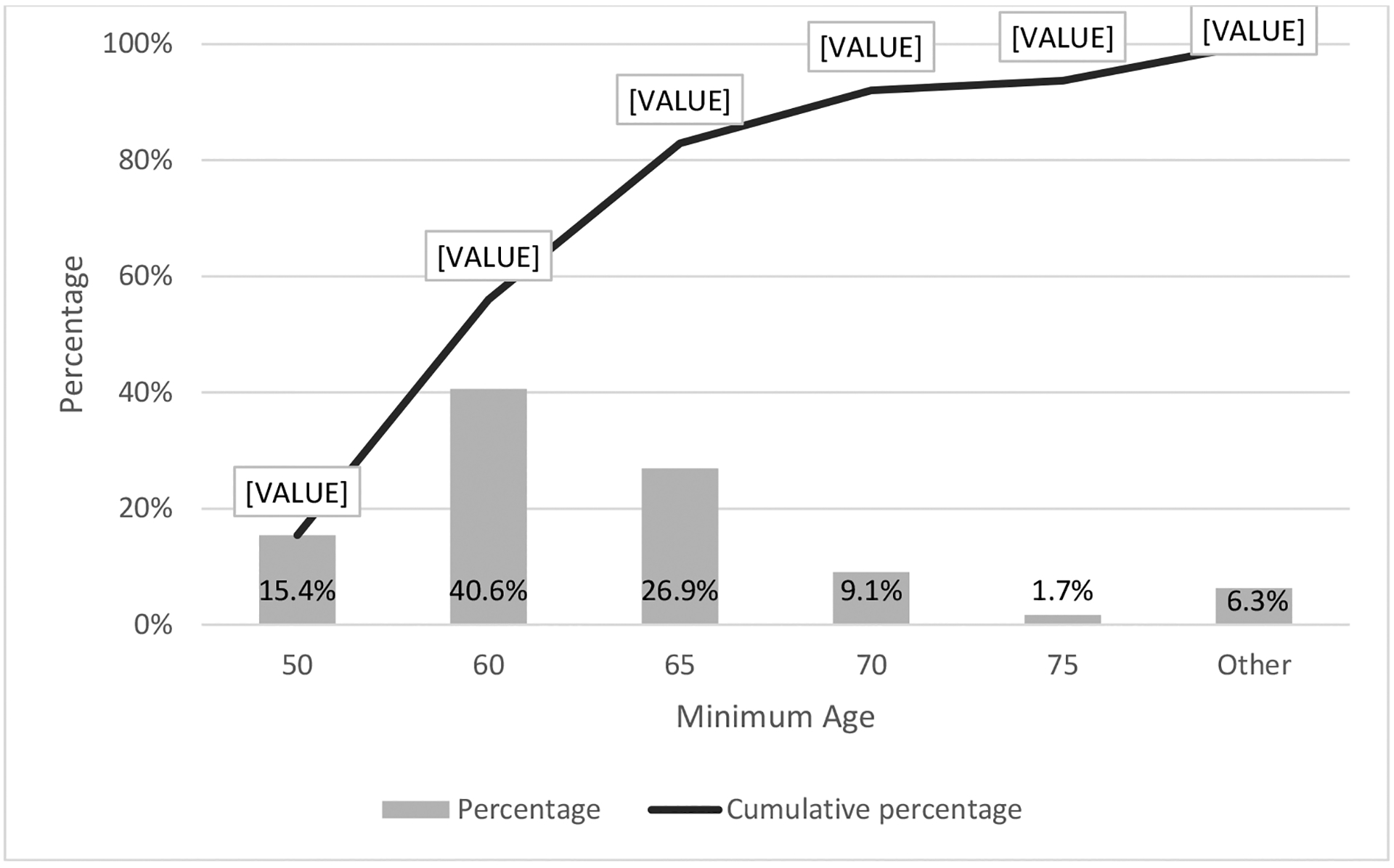
Minimum patient age in years at which physicians believed a standardized toolkit to evaluate alloHCT candidacy is most critical (n=175)
*Other responses included: “All ages” (n=5) and “Other” [not specified] (n=6)
Impact of Geriatric Evaluation and Assessment on Treatment Decisions and Perceived Barriers
More than three-quarters of physicians noted that their centers rarely or never (n=138, 79%) utilize a dedicated geriatrician or geriatric-oncologist to assess older alloHCT candidates. Of those physicians whose centers utilized a dedicated specialist (n=37, 21%), only 5% (n=8) reported using this specialist all of the time.
Nearly half of all transplant physicians, (45%, n=78) reported that GA routinely impacted the decision for transplant versus no transplant; other transplant-related decisions were impacted including utilization of MAC versus RIC/NMA (n=64, 37%), and supportive care measures (n=60, 34%), with nearly a third of physicians (34%, n=59) describing no impact of GA on their treatment decisions.
Figure 5 shows pre-specified barriers to performing a GA for older adults in this context, The three most common barriers included: uncertainty about which assessment tools to use (n = 132, 76% strongly agree or agree); lack of training, knowledge, understanding or experience about geriatric assessment (n=125, 72% strongly agree or agree) and lack of clinical support staff (n=118, 68% strongly agree or agree).
Figure 5.
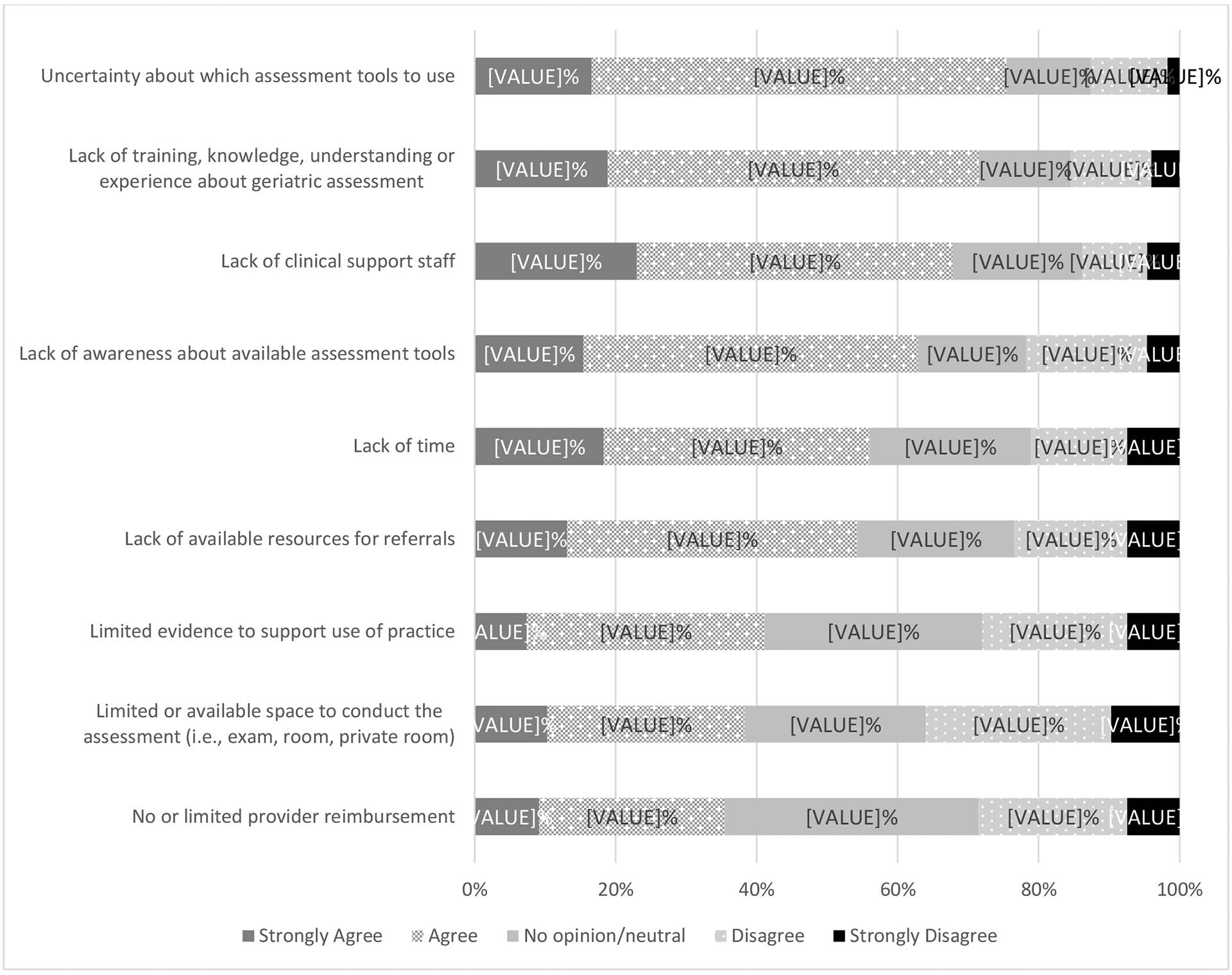
Agreement with pre-specified barriers to performing geriatric assessment for patients age 60 years and older.
Note: n=175 for all barriers, except n=174 for “lack of clinical support”
The only baseline physician characteristic significantly associated with perceiving use of GA to influence the decision for transplant versus non-transplant was younger age of the physician. Adjusting for other characteristics (gender, practice type, years of practice, and center volume), physicians aged 41–60 and ≥61 years were less likely than younger physicians (age 30–40) to indicate GA impacted treatment decisions (41–60 vs. 30–40: odds ratio [OR]=0.34, 95% confidence interval [CI]: 0.12–1.0, p=0.05; ≥61 vs. 30–40: OR=0.18; 95% CI: 0.04–0.76, p=0.02).
DISCUSSION
To our knowledge, this is the first study to comprehensively examine transplant physicians’ perceptions of older adult candidacy for alloHCT. While guidelines exist for assessment of older general oncology patients, established guidelines for this population undergoing HCT are currently unavailable.(15, 16) The striking heterogeneity by which transplant physicians assess candidacy and treatment decisions for older adults substantiates the need to standardize characterization of patient health. Nevertheless, over 90% of alloHCT physicians will consider alloHCT in patients up to the age of 75 years and not uncommonly, in patients older than that. While the validity and implementation of age limits in actual practice cannot be determined, registry data confirms markedly increasing utilization of alloHCT in older adults as well as improved survival in patients 70 years and older.(6) Notably, a minority of physicians reported consideration of MAC in patients above 65 years and a substantial proportion noting no UAL when considering RIC, underscoring the willingness of transplant physicians to entertain different alloHCT approaches for older adults in an era of improved transplant supportive care.
While patient age itself did not preclude all transplant physicians from considering alloHCT into the eight decade, older age persists as the largest barrier to alloHCT for standard indications. In one report, only 17% of patients 60 years and older with AML were offered alloHCT.(7) Likewise, a National Cancer Database report revealed only 5.5% of patients with AML between the ages of 61–75 ultimately underwent alloHCT although 42% of new AML occurs from ages 55–74 (https://seer.cancer.gov/statfacts/html/amyl.html).(4) Numerous biologic and non-biologic factors impact transplant referral and candidacy; however, significantly increased odds for HCT non-referral based on advanced chronologic age alone has been previously described.(9, 17) Our data on willingness of HCT physicians to consider transplantation for older patients suggests lack of referral to a transplant center as a significant obstacle consistent with data demonstrating older age as the primary impediment to HCT referral from community oncologists.(17)
As we enter an era of routine deliberation of alloHCT for older adults, risk stratification based on comprehensive health profiling holds promise. Most physicians (66%) agreed that the standard transplant assessments of KPS and HCT-CI reflect overall functional status and health, respectively, and may even employ thresholds for candidacy. This is not surprising as these are standard tools, but a perceived lack of their ability to fully predict outcomes of HCT for older patients may explain physician interest in exploring additional tools to address candidacy. Nevertheless 90% of physicians reported a need for a specialized assessment toolkit beyond KPS and HCT-CI to better address HCT candidacy. GA, a multi-dimensional health assessment tool, uncovers functional limitations and frailty prior to alloHCT in patients 50 years and older and risk-stratifies for outcomes.(14, 19) Our study demonstrates that GA is not routinely adopted at the participating physicians’ HCT centers (45%), and geriatricians or geriatric oncologists engagement occurs even less so (21%). The lack of geriatric trained collaborators aligns with the reliance by the transplant physicians on function and co-morbidity as a crude measure for health assessment. Unfortunately, KPS and HCT-CI fail to identify additional aging-related vulnerabilities regularly evaluated in a GA such as cognition, falls, weight loss and emotional health. The perceived barriers to performing GA are high, similar to a recent American Society of Clinical Oncology (ASCO) survey of cancer providers.(20). Our study quantified barriers to routine GA among older alloHCT candidates, including lack of clinical support staff; uncertainty about which assessment tools to use; lack of training, knowledge, understanding or experience with GA; and lack of time. While lack of time was the most common barrier noted in the ASCO study, transplant physicians reported uncertainty and lack of training or knowledge on GA tools as the most common barriers, similar to ASCO respondents who were not aware of ASCO GA guidelines. Still, the majority perceived a need for supplemental information available on older patients’ health before alloHCT. This finding highlights the need for clarity on recommended assessment and/or tools for older alloHCT patients.
Finally, we found younger physicians more frequently incorporated GA in treatment decisions for older patients. We hypothesize introduction of geriatrics into internal medicine training programs in 1994, improving over time,(21) enhanced younger physicians geriatric comfort and content knowledge.
The lack of prospective studies on the prognostic relevance of health assessments tools such as GA may account for variability in application found herein. A Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 1704 (https://clinicaltrials.gov/ct2/show/NCT03992352) prospective multi-center observational study aims to evaluate pre-transplant GA and other factors that predict NRM in alloHCT recipients 60 years or older. Although our survey occurred prior to initiation of CTN 1704, awareness of the CTN study may have influenced physician responses. Based on prior and ongoing studies, we envision GA will be recommended for pre-HCT assessment in older patients. Nevertheless, our data indicate additional barriers to implementation may exist. Our survey queried on domains evaluated (e.g., function, cognition) rather than specific tools, precluding estimates on the utilization frequency of specific instruments. We further believe the rich data from GA should not simply be another marker for candidacy, but rather to encourage interventions to reduce complications.(22) The data suggest interventions targeted to GA-defined vulnerabilities are infrequent, as most respondents rarely or never utilized a dedicated geriatrician or geriatric-oncologist to evaluate older alloHCT candidates. Additional studies are needed to establish the value of inclusion of geriatric-trained providers.
This study has several limitations. While the modest response rate potentially limits the generalizability of our findings, the response rate mirrored other physician surveys.(23, 24) Respondents with a special interest in aging and HCT may have been more inclined to take the survey although we excluded from the survey the subset of physicians engaged in questionnaire development. We did not capture actual transplant center practices; we anticipate physician response may be aligned with center practice and policies. Anonymous survey responses precluded comparison of characteristics of responders to non-responders, and we were unable to assess how many individual transplant centers were represented by the respondents.
CONCLUSION
Transplant physicians commonly consider alloHCT in patients over the age of 70 years. Therefore, community physician perception of age alone as candidacy for alloHCT is an important modifiable barrier that could help facilitate early referral to transplant center. HCT physicians’ application of tools and domains varies widely in assessing older adults for their risk for transplant-related morbidity. Incorporation of a standardized pre-transplant health assessment tool for risk stratification is a significant unmet need. Future efforts to facilitate incorporation of GA into pre-HCT clinical care may ultimately reduce age-related barriers to alloHCT.
Supplementary Material
Highlights.
Many physicians will consider alloHCT in patients up to age 75 years (and older).
Heterogeneity exists in evaluating pre-HCT health status and alloHCT candidacy.
A standardized health assessment to predict outcomes for older adults is needed.
Acknowledgements:
The authors wish to thank all respondents for their insights. The authors also thank Monique Ammi, Clinical Research Coordinator I, and the Health Services Research team at the National Marrow Donor Program/Be The Match, who provided assistance with survey administration and survey draft review. The authors also wish to thank Dr. William Dale, MD, PhD Arthur M. Coppola Family Chair in Supportive Care Medicine and the Director of the Center for Cancer Aging Research at the City of Hope, Duarte, California for his subject matter expertise.
Disclosure:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; HHSH250201700006C, SC1MC31881-01-00 and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612 and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, Japan Hematopoietic Cell Transplantation Data Center, St. Baldrick’s Foundation, the National Marrow Donor Program, the Medical College of Wisconsin and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Adienne SA; Allovir, Inc.; Amgen, Inc.; Angiocrine Bioscience; Anthem, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Gamida-Cell, Ltd.; Genzyme; HistoGenetics, Inc.; Incyte Corporation; Janssen Biotech, Inc.; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Medac GmbH; Merck & Company, Inc.; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; OptumHealth; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; REGiMMUNE Corp.; Sanofi Genzyme; Shire; Sobi, Inc.; Takeda Pharma; Terumo BCT; Viracor Eurofins; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Financial conflicts of interest to report:
Asmita Mishra reports research funding from Novartis. Vijaya Raj Bhatt reports receiving consulting fees from Partner Therapeutics, CSL Behring, Genentech, Rigel, Agios, Incyte, Omeros, Takeda, Partnership for health analytic research, LLC (which in turn, receives funds from Jazz Pharmaceuticals) and Abbvie, research funding (institutional) from Abbvie, Pfizer, Incyte, Jazz, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program, drug support (institutional) from Oncoceutics for a trial, and educational grant (institutional) from Pfizer and Novartis. Anita D’Souza reports Grant support: K23 HL141445, Research funding: Takeda, Sanofi, TeneoBio, Janssen; Ad Board: Imbrium, Akcea, Pfizer; Consulting: Janssen. Parastoo B. Dahi reports serving on the advisory board for Kite/Gilead. Rebecca Olin reports research support from: Astellas, Daiichi Sankyo, Genentech, Pfizer, and consulting support from: Genentech, Jazz Pharmaceuticals, Amgen. Mohamed Sorror reports advisory committee membership role and honorarium from JAZZ Pharmaceuticals in 2019. Anthony Sung reports research funding from Merck, Novartis, Seres; speaker’s honorarium from Abbott, and consult for AVROBIO, all outside of the submitted work. Celalettin Ustun reports honoraria (because of attending advisory board) from Novartis and Blueprint, outside the submitted work. William A. Wood reports research funding from Pfizer, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
No financial conflicts of interest to report:
Jaime Preussler, Christopher Bredeson, Saurabh Chhabra, Eileen Danaher Hacker, Lohith Gowda, Shahrukh K. Hashmi, Dianna S. Howard, Ann Jakubowski, Reena Jayani, Thuy Koll, Richard J. Lin, Uday Popat, Cesar Rodriguez, Ashley Rosko, Mitchell Sabloff, Linda Burns, Andy Artz have no financial conflicts of interest to report.
REFERENCES
- 1.D’Souza AFC. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides 2019. [Available from: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.
- 2.D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2020;26(8):e177–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasu S, Kohlschmidt J, Mrózek K, Eisfeld A-K, Nicolet D, Sterling LJ, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Advances. 2018;2(13):1645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt VR, Chen B, Gyawali B, Lee SJ. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplantation. 2018;53(10):1288–94. [DOI] [PubMed] [Google Scholar]
- 5.Wall SA, Devine S, Vasu S. The who, how and why: Allogeneic transplant for acute myeloid leukemia in patients older than 60years. Blood Reviews. 2017;31(6):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130(9):1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorror ML, Estey E. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in older adults. Hematology American Society of Hematology Education Program. 2014;2014(1):21–33. [DOI] [PubMed] [Google Scholar]
- 8.Artz AS. From biology to clinical practice: aging and hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2012;18(1 Suppl):S40–5. [DOI] [PubMed] [Google Scholar]
- 9.Pidala J, Craig BM, Lee SJ, Majhail N, Quinn G, Anasetti C. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. 2013;48(1):63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. The Lancet Oncology. 2018;19(6):e305–e16. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29(25):3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro PD, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2013;19(3):429–34. [DOI] [PubMed] [Google Scholar]
- 15.Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of Clinical Oncology. 2018;36(22):2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NCCN Guidelines for Older Adult Oncology [Available from: https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=1445.
- 17.Meyer C, Mau LW, Murphy EA, Denzen EM, Hayes E, Haven D, et al. Addressing Knowledge Gaps in Acute Myeloid Leukemia to Improve Referral for Hematopoietic Cell Transplantation Consultation. Journal of the National Comprehensive Cancer Network: JNCCN. 2019;17(12):1473–81. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson B, de Lima M, Cogle CR, Thompson MA, Grinblatt DL, Pollyea DA, et al. Transplant Referral Patterns for Patients (Pts) with Newly Diagnosed (ND) Higher-Risk (HR) Myelodysplastic Syndromes (MDS), and European Leukemianet (ELN) 2010 Intermediate-Risk (IR) or Adverse-Risk (AR) Acute Myeloid Leukemia (AML) in the Connect® MDS/AML Registry. Biology of Blood and Marrow Transplantation. 2020;26(3, Supplement):S98–S9. [Google Scholar]
- 19.Huang LW, Sheng Y, Andreadis C, Logan AC, Mannis GN, Smith CC, et al. Functional Status as Measured by Geriatric Assessment Predicts Inferior Survival in Older Allogeneic Hematopoietic Cell Transplantation Recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2020;26(1):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale W, Williams GR, R. MacKenzie A, Soto-Perez-de-Celis E, Maggiore RJ, Merrill JK, et al. How Is Geriatric Assessment Used in Clinical Practice for Older Adults With Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncology Practice. 2020:OP.20.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahnigen D Geriatric education for internal medicine residents. The American Journal of Medicine. 1994;97(4a):43s–5s. [DOI] [PubMed] [Google Scholar]
- 22.Derman BA, Kordas K, Ridgeway J, Chow S, Dale W, Lee SM, et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Advances. 2019;3(22):3488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Jawahri A, LeBlanc TW, Burns LJ, Denzen E, Meyer C, Mau L-W, et al. What do transplant physicians think about palliative care? A national survey study. Cancer. 2018;124(23):4556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann JL, Mau LW, Virani S, Denzen EM, Boyle DA, Boyle NJ, et al. Burnout, Moral Distress, Work-Life Balance, and Career Satisfaction among Hematopoietic Cell Transplantation Professionals. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2018;24(4):849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


