Figure 4. WNK1 binds the hydrophobic EMC2-EMC8 interface.
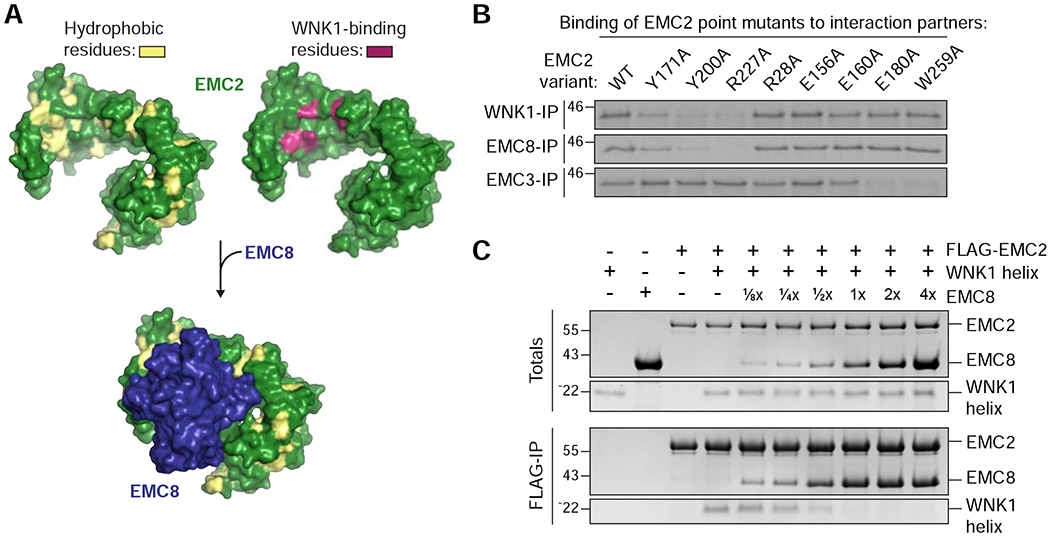
(A) Depicted is the surface representation of EMC2 (PDB 6WW7; Pleiner et al., 2020) in which hydrophobic residues are highlighted in yellow (left) and mutations that affected WNK1 binding are highlighted in purple (right, see B). The WNK1 binding site overlapped with the binding site of EMC8 (blue), which is characterized by an extended hydrophobic patch. EMC2 is viewed from the cytosol. (B) 35S-methionine-labeled wild type (WT) EMC2, or the indicated point mutants were translated in RRL and tested for binding to 3xFLAG-tagged WNK1, EMC8 or EMC3 via co-immunoprecipitation using anti-FLAG resin. EMC2 mutants that are unable to bind WNK1 also do not bind EMC8, but are capable of interacting with EMC3, indicating proper folding. (C) A stoichiometric complex of purified FLAG-tagged EMC2 and WNK1 helix was pre-formed on ice and then incubated with increasing amounts of purified EMC8. The resulting EMC2 complexes were then immunoprecipitated with FLAG resin and analyzed by SDS-PAGE and Coomassie staining.
