Figure 6. WNK1 prevents EMC2 ubiquitination and directly competes for E3 ligase binding.
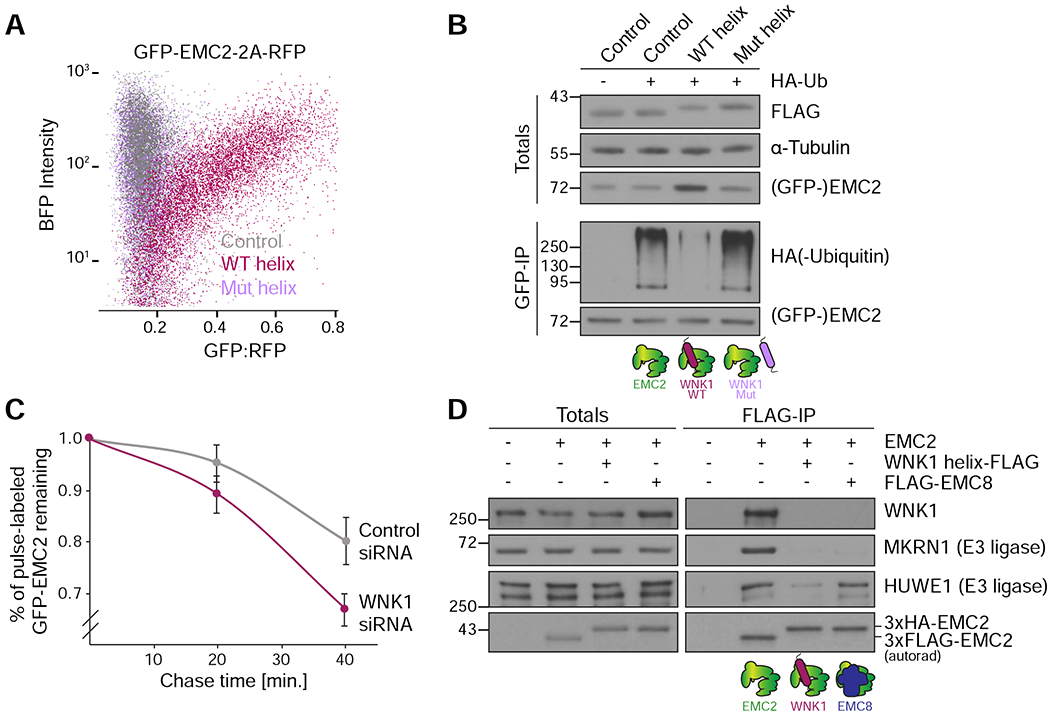
(A) HEK293T cells stably expressing GFP-EMC2 were transfected with plasmids encoding BFP (control), BFP-tagged WNK1 wild type (WT) or L2250K mutant (Mut) helix. Cells were analyzed by flow cytometry and their relative GFP intensity, normalized to an internal RFP expression control, was plotted against BFP intensity.(B) Stable HEK293T GFP-EMC2 cells were transfected with plasmids encoding BFP (control), BFP-tagged WNK1 WT or Mut helix −/+ HA-tagged ubiquitin. Cells were additionally treated with 10 nM bortezomib for 16 hours before harvest. Unassembled GFP-EMC2 was purified from cell lysate prepared in the presence of 50 μM deubiquitinase inhibitor PR-619 using an anti-GFP nanobody (as in Figure 1D), and eluates were normalized by GFP fluorescence. Samples of total lysates and eluates were analyzed by SDS-PAGE and Western blotting with the indicated antibodies. (C) Stable HEK293T GFP-EMC2 cells were treated with either scrambled or WNK1 siRNA, and newly synthesized proteins were pulse-labeled with 35S-methionine. Following a chase with excess unlabeled methionine, the amount of remaining labeled GFP-EMC2 was assessed after 0, 20 and 40 minutes by immunoprecipitation with an anti-GFP nanobody. Eluates were normalized by GFP fluorescence and then analyzed by SDS-PAGE and autoradiography (n=3). (D) 35S-methionine-labeled EMC2 was translated in RRL and purified either directly via a 3xFLAG tag or when bound to 3xFLAG-WNK1 helix or 3xFLAG-EMC8 (in those cases 3xHA-tagged EMC2 was utilized). Samples of totals and eluates were analyzed by SDS-PAGE and Western blotting with the indicated antibodies. EMC2 was detected by autoradiography (autorad). Binding of the co-purifying E3 ligases MKRN1 and HUWE1, identified by mass spectrometry (Figure 1C, Table S2), to EMC2 is incompatible with binding to WNK1.
