Figure 7. Model for assembly of the cytosolic domain of the EMC.
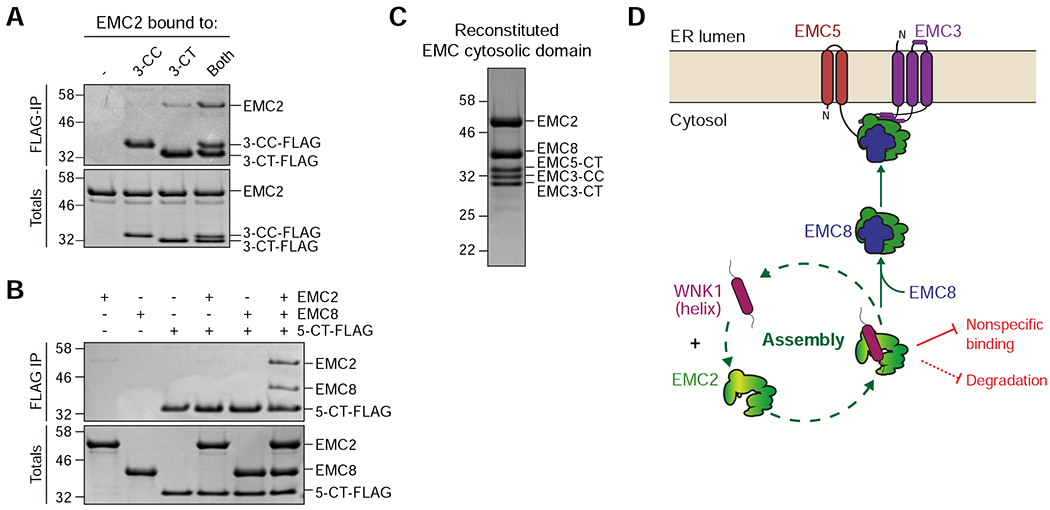
(A) Both the EMC3 coiled coil (3-CC) and C-terminus (3-CT) are needed to interact with EMC2. Purified 3xFLAG-tagged 3-CC and 3-CT were incubated with EMC2 either alone or in combination and then subjected to FLAG-IP. Eluates were analyzed by SDS-PAGE and Coomassie staining. (B) The EMC5 C-terminus (5-CT) selectively binds the EMC2-8 complex. 3xFLAG-tagged 5-CT was incubated either with buffer, EMC2, EMC8, or both and subjected to FLAG-IP. Totals and eluates were analyzed by SDS-PAGE and Coomassie staining. (C) Reconstitution of the EMC cytosolic domain from purified components: EMC2, EMC8, EMC3-coiled coil (CC), EMC3-C-terminus (CT) and EMC5-CT. The complex was analyzed by size-exclusion chromatography (SEC), followed by SDS-PAGE and Coomassie staining of the peak fraction. (D) WNK1 stabilizes unassembled EMC2 by using a conserved C-terminal amphipathic α-helix to prevent nonspecific binding and premature degradation in the cytosol. WNK1 exerts these protective functions by shielding a hydrophobic patch on EMC2 at the EMC2-8 interface and by directly competing with E3 ligase binding to EMC2, strongly reducing its ubiquitination. EMC8 then displaces WNK1 in the cytosol, allowing assembly of the EMC2-8 complex with the membrane-bound subunits EMC3 and EMC5.
