Fig. 7 |. Molecular mechanisms of anti-cancer AuQC705–BAMLeT lipoprotein nanocomplex.
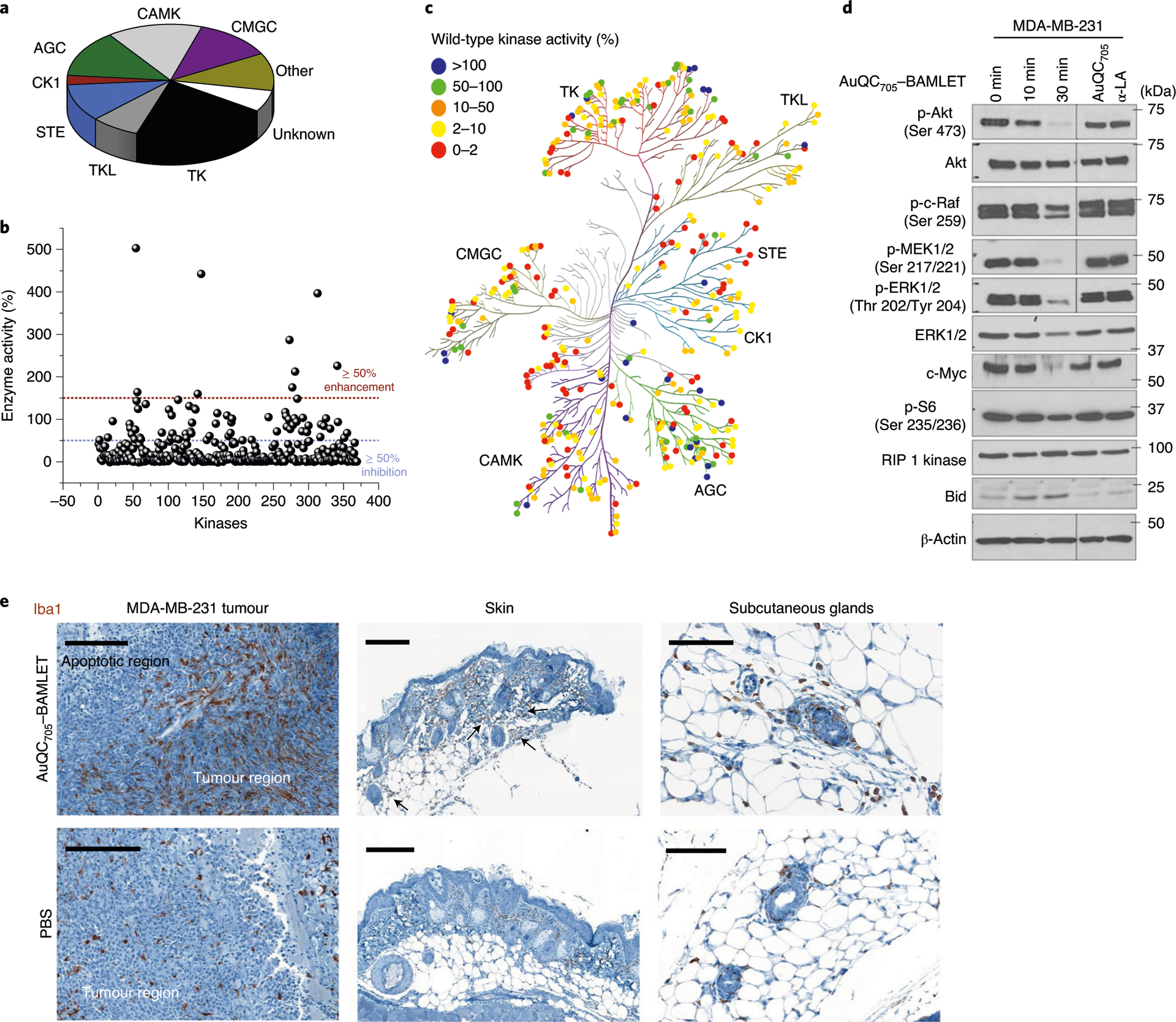
a, The distribution of all of the wild-type human kinases in the screening panel. They are classified into nine groups as follows: protein kinases A, G and C (AGC), calmodulin/calcium-dependent kinases (CAMK), casein kinase 1 (CK1), CDK, MAPK, GSK3 and CLK (CMGC), homologues of yeast sterile 7, 11 and 20 (STE), tyrosine kinase (TK), tyrosine kinase-like (TLK), other (not belong to any of the above kinase families) and unknown (kinase–partner complexes and isoforms that could not be mapped on kinome tree). b, Kinase inhibition by AuQC705–BAMLET. Wild-type human kinases can be broadly inhibited (≥ 50% inhibition). c, Human kinome dendrographic mapping of kinase targets of AuQC705–BAMLET. Each dot in the dendrogram indicates an individual kinase and each branch represents a kinase group. The strongest inhibition is shown in red and enhancement is shown in blue. d, Western blots of molecules involved in cell-death regulatory signalling pathways. β-Actin was used as the internal loading control. Molecular mass markers are labelled on the right. p-, phosphorylated protein. e, IHC staining of macrophage-specific ionized calcium-binding adapter molecule 1 (Iba1) for MDA-MB-231 breast tumours, skin tissues and enlarged subcutaneous glands. Tissues were resected from J:NU xenograft mice 24 h after intratumoural or subcutaneous injection of AuQC705–BAMLET (150 μg) or PBS. Macrophage-enriched areas of subcutaneous glands are indicated by black arrows. Scale bars, 200 μm (left and middle) and 100 μm (right).
