Figure 2 .
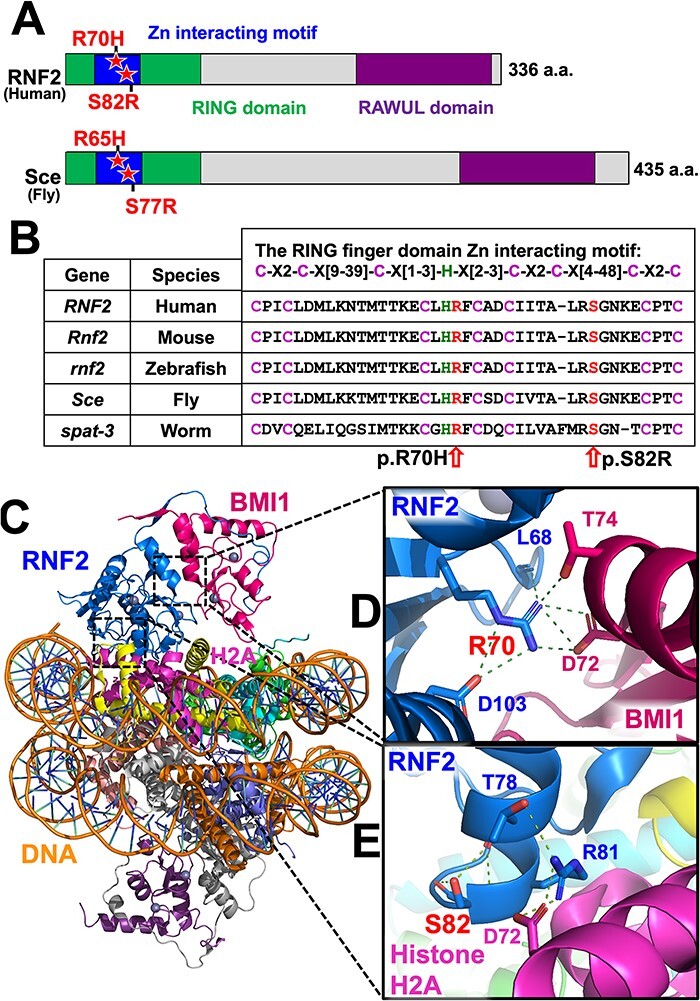
p.R70H and p.S82R variants affect evolutionarily conserved amino acids within the RING finger domain Zn interacting motif that is crucial for molecular interactions in PRC1. (A) Domain structure of human RNF2 and Drosophila Sce proteins. Variants identified in the Patients (p.R70H and p.S82R) and their corresponding amino acids in the fly protein are shown with a star. (B) Sequence alignments of the RING finger domain Zn2+ interacting motif of RNF2 orthologs in human (Homo sapiens), mouse (Mus musculus), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster) and worm (Caenorhabditis elegans). (C) Structural model of the Polycomb repressive complex 1 (PRC1, PDB ID: 4R8P) highlighting RNF2 in blue, BMI1 in purple, Histone H2A in pink and DNA in orange. The backbone of DNA is shown orange. (D) Arginine 70 (p.R70) interaction network at RNF2 and BMI1 interface with key residues shown in sticks presentation. The hydrogen bonds and salt bridges are shown as green dotted lines connecting to p.R70. (E) Serine 82 (p.S82) which forms hydrogen bonds to stabilize the α-helix as a capping residue and Arginine 81 (p.S81) interaction network at RNF2 and Histone H2A interface with key residues shown in sticks presentation. The hydrogen bonds and salt bridges are shown as green dotted lines. RING, really interesting new gene; RAWUL, ring finger and WD40 ubiquitin-like).
