Abstract
Endometrial cancer (EC) ranks as the fourth most common diagnosed cancer type in females worldwide. MicroRNAs (miRNAs) are important regulators with crucial roles in regulating diverse biologic processes, including tumor initiation and progression. Previous studies have demonstrated that miR-27a was correlated with the tumorigenesis of various cancers. However, its expression and biologic role in EC remain to be determined. This study aimed to clarify whether miR-27a acts as an oncogene in endometrial cancer (EC) by downregulating ubiquitin specific peptidase 46 (USP46). Expression of miR-27a was measured by qRT-PCR, and the results demonstrated that miR-27a was upregulated in EC cell lines compared to normal cell lines. Cell counting kit-8 (CCK-8) and wound-healing assays demonstrated that overexpression of miR-27a significantly promoted cell proliferation and migration. Online prediction algorithm and dual luciferase activity reporter assay revealed that USP46 acts as a direct target of miR-27a. USP46 expression was downregulated in EC cell lines during miR-27a overexpression. Collectively, our results indicated that miR-27a targets USP46 to promote EC cell proliferation and migration, suggesting an oncogene role of miR-27a in EC.
Keywords: miR-27a, USP46, endometrial cancer, oncogenic miRNA
Introduction
Endometrial cancer (EC) is the most common type of gynecologic cancer [1]. Most EC patients can be diagnosed at early stages; however, about 30% will experience metastasis [2]. The treatment for EC has improved greatly in recent years but some have poor prognosis [3]. Hence, identifying the molecules that contribute to the progression of EC is essential.
microRNAs (miRNAs) are non-coding RNAs that regulate gene expression via promoting message RNA degrading or repressing translation [4]. Numerous miRNAs have been identified to be correlated with the progression of EC [5]. For instance, miR-215 was found upregulated in EC and its overexpression was able to promote epithelial to mesenchymal transition and colony formation by targeting the expression of left-right determination factor 2, indicating an oncogenic role of miR-215 in EC [6]. Many studies illustrate the tumor suppressive role of miRNAs in EC. miR-139-5p was found downregulated in EC tissues and its overexpression inhibits cancer growth and migration through regulating the expression of HOXA10 [7]. miR-148b was also found downregulated in EC to repress cell growth by regulating hypoxia-inducible factor 1 and nuclear factor erythroid 2-related factor 2 by downregulating endoplasmic reticulum metalloprotease 1 [8]. miR-27a was previously reported to function as an oncogene in human cancers including ovarian cancer and lung cancer [9,10]. However, its expression and biologic role in EC remains unclear.
Ubiquitin specific peptidase 46 (USP46) is a deubiquitinating enzyme that controls androgen receptor and P53 pathways in prostate cancer, indicating targeting this deubiquitinating enzyme is a potential treatment strategy for prostate cancer [11]. In colon cancer, USP46 shown to stabilize the expression of PH domain leucine-rich-repeats protein phosphatase to inhibit cancer cell proliferation and tumorigenesis, indicating the tumor suppressive role of USP46 [12]. It was not determined whether USP46 has a role in EC progression.
In this study, we found miR-27a expression was upregulated in EC cell lines, and its knockdown inhibits EC cell proliferation and migration. Bioinformatic analysis, luciferase activity reporter assay, and western blot validated USP46 as a direct target of miR-27a. USP46 knockdown promoted EC cell proliferation, migration, and partially reversed the effects of miR-27a knockdown on EC cell behavior.
Materials and methods
Cell lines and cell transfection
EC cell lines (HEC-1A and HEC-1B) and normal endometrial cell line (EMC) were purchased at Cell Bank of Chinese Academy of Sciences (Shanghai, China). These cells were cultured in DMEM (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) at 37°C humidified incubator containing 5% of CO2.
miR-27a inhibitor and the negative control (NC-miR) were synthesized by RiboBio (Guangzhou, China). Small interfering RNA targeting USP46 (si-USP46) and the negative control (NC-siR) were also purchased from RiboBio. Cell transfection was conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 48 h of transfection, cells were collected for following analyses.
Quantitative real-time RT-PCR (qRT-PCR) analysis
Total RNA was extracted from EC cells using TRizol reagent (Invitrogen) according to the manufacturer’s protocol. Complementary DNA was reverse transcribed from RNA using PrimeScript RT Reagent kit (Takara, Dalian, Liaoning, China). qRT-PCR was conducted using SYBR Green PCR kit (Takara) at an Applied Biosystems 7300 System (Applied Biosystems, Foster City, CA, USA). Relative expression of miR-27a was normalized to U6 snRNA using the 2-ΔΔCt method. The detailed procedures were as follows: 1 cycle of 95°C 10 min; 40 cycles of 95°C 15 s, 67°C 30 s, 72°C 30 s. The primers used are: miR-27a: F: 5’-TGCGGTTCACAGTGGCTAAG-3’, R: 5’-CTCAACTGGTGTCGTGGA-3’; U6 snRNA: F: 5’-CTCGCTTCGGCAGCACA-3’, R: 5’-AACGCTTCACGAATTTGCGT-3’.
Western blot analysis
EC cells were lysed in radioimmunoprecipitation assay lysis buffer supplemented with protease and phosphatase inhibitor (Beyotime, Haimen, Jiangsu, China). Total protein was isolated at 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membrane (Invitrogen). After blocking with fat-free milk, membranes were incubated overnight at 4°C with goat anti-rabbit primary antibodies (anti-USP46: ab88795; anti-GAPDH: ab8245; Abcam, Cambridge, MA, USA). Then, blot was incubated with HRP conjugated goat anti-mouse secondary antibody (ab6789, Abcam) at room temperature for 1 h, and visualized using BeyoECL kit (Beyotime).
Cell counting kit-8 (CCK-8) assay
Cell proliferation rate was measured using CCK-8 kit (Beyotime). After seeding the cells in 96-well plates at the density of 1.0 × 103 cells/well and incubated for indicated time points, 10 μl of CCK-8 reagent was added and incubated for additional 4 h. Optical density was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Transwell migration assay
Migration assays of EC cells were conducted using Transwell chambers. 1 × 105 cells in serum-free DMEM were placed into the top chamber. DMEM contains FBS was filled into the bottom chamber. After the incubation for 24 h, cells were fixed with polyoxymethylene, stained with crystal violet, and we counted five random fields under a microscope.
Dual-luciferase reporter assay
TargetScan was employed to analyze the putative target of miR-27a. The 3’-UTR of USP46 with the putative target sequence for miR-27a was cloned and inserted at pGL3-control (Promega, Madison, WI, USA) and named as USP46-wt. The mutant luciferase vector was built from USP46-wt using Site-direct mutagenesis kit (Takara) and then designated at USP46-mt. Cells were co-transfected with miRNAs and luciferase vectors using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured 48 h after transfection using Dual luciferase assay system (Promega).
Statistical analysis
Results were presented as the mean ± standard deviation after analyzed at SPSS 19.0 software (Chicago, IL, USA). Differences in groups were analyzed with paired Student’s t-test or one-way analysis of variance (ANOVA) and Tukey post-hoc test. P value less than 0.05 was considered a significant difference.
Results
Upregulation of miR-27a in EC cell lines
To determine the role of miR-27a in EC progression, we examined its expression in EC cell lines (HEC-1A and HEC-1B) and a normal endometrial cell line (EMC). miR-27a expression was significantly upregulated in EC cell lines investigated compared to that in EMC cell line (Figure 1).
Figure 1.

miR-27a was upregulated in EC cell lines (HEC-1A and HEC-1B) compared with normal endometrial cell line (EMC). miR-27a: microRNA-27a; EC: endometrial cancer.
Knockdown of miR-27a inhibits EC cell proliferation and migration
To assess the effect of miR-27a in EC, the EC cell lines were transfected with synthetic miRNAs. Compared with the NC-miR group, miR-27a expression was significantly reduced by miR-27a inhibitor (Figure 2A). CCK-8 assay showed that downregulation of miR-27a significantly inhibited proliferation of EC cells (Figure 2B). Moreover, the migration assay showed that cell migration ability was significantly suppressed by miR-27a inhibitor (Figure 2C).
Figure 2.

Knockdown of miR-27a decreased EC cell proliferation and migration. (A) miR-27a expression, (B) Cell proliferation, and (C) Cell migration in EC cells transfected with miR-27a inhibitor or NC-miR. miR-27a: microRNA-27a; EC: endometrial cancer; NC-miR: negative control miRNA.
USP46 was a direct target of miR-27a
Binding site of miR-27a and the 3’-UTR of USP46 was displayed in Figure 3A. Figure 3B demonstrated that luciferase activity was very increased in cells co-transfected with miR-27a inhibitor and USP46-wt compared to those co-transfected with miR-27a inhibitor and USP46-mt. Western blot revealed that USP46 expression was significantly increased by miR-27a inhibitor (Figure 3C).
Figure 3.

USP46 was a candidate gene of miR-27a. A. Computational analysis identified that USP46 may be a target of miR-27a. B. Dual luciferase assay performed in EC cells confirmed USP46 was a target of miR-27a. C. USP46 expression in EC cells transfected with miR-27a inhibitor or NC-miR. miR-27a: microRNA-27a; EC: endometrial cancer; NC-miR: negative control miRNA; USP46: ubiquitin specific peptidase 46; wt: wild-type; mt: mutant; UTR: untranslated region.
Knockdown of USP46 promotes EC cell proliferation and migration
Expression of USP46 was significantly decreased by si-USP46 as compared with NC-siR (Figure 4A). We also found the cell proliferation and migration of EC cells was significantly elevated after miR-27a knockdown (Figure 4B and 4C). In addition, we found the effects of miR-27a on EC cell proliferation and migration were partially reversed by USP46 (Figure 4B and 4C).
Figure 4.
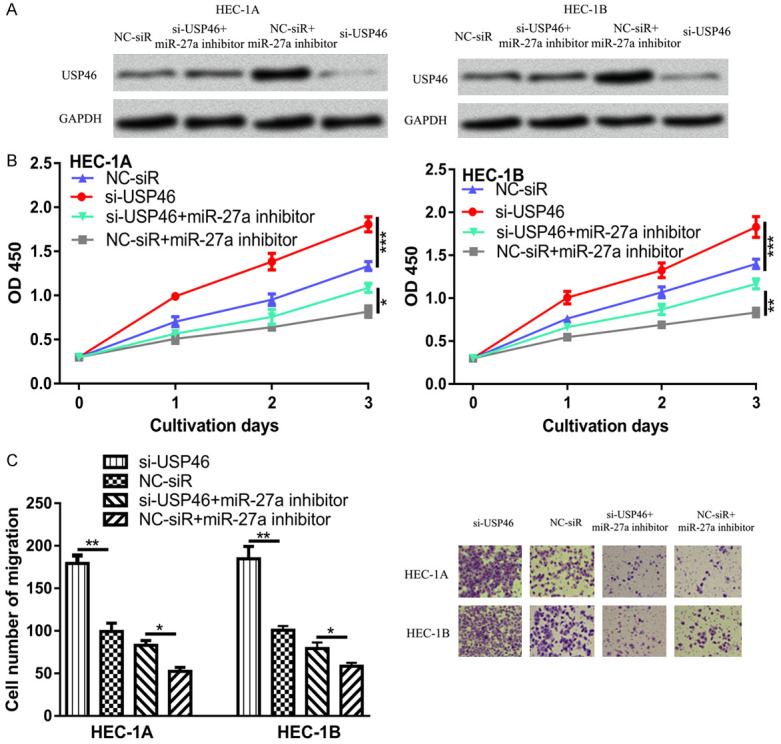
USP46 knockdown promotes EC cell proliferation and migration. (A) USP46 expression, (B) Cell proliferation, and (C) Cell migration in EC cells transfected with si-USP46, NC-siR, or si-USP46 and miR-27a inhibitor. miR-27a: microRNA-27a; EC: endometrial cancer; USP46: ubiquitin specific peptidase 46; NC-siR: negative control small interfering RNA; si-USP46: small interfering RNA targeting USP46.
Discussions
In the present study, we showed that miR-27a expression was upregulated in EC cell lines investigated compared to the normal cell line. We showed that knockdown of the expression of miR-27a was able to inhibit EC cell proliferation and migration. Mechanically, we screened USP46 as a potential target for miR-27a using TargetScan. The knockdown of USP46 dramatically increased EC cell proliferation and migration. The luciferase activity reporter assay and western blot revealed that USP46 was a direct target of miR-27a. Functional assays revealed that knockdown of expression of USP46 partially reversed the effects of miR-27a on EC cell behaviors in vitro, indicating USP46 was a functional target of miR-27a.
miR-27a has been reported to be upregulated in multiple human cancers including ovarian cancer, lung cancer, and colorectal cancer, functioning as a tumor promoter [9,10,13,14]. Although our results also showed that miR-27a functions as oncogenic miRNA in EC as reported previously, the molecular mechanisms are different. In ovarian cancer, Zhang et al. showed that miR-27b could promote migration, invasion, and EMT of cancer cell by the Wnt/β-catenin signaling pathway and resulted in FOXO1 expression inhibition [9]. In lung cancer, miR-27b was shown able to decrease SMAD2 and SMAD4 expression by TGF-β signaling pathway [10]. In colorectal cancer, high miR-27a expression was correlated with poor overall survival, advanced tumor stage, and worse distant metastasis of cancer patients [13]. Our results discovered a novel miR-27a/USP46 axis in cancer progression, which explains the crucial role of miR-27a in cancers.
Although non-coding RNAs have shown to be crucial regulators in cancers [15-17]. However, the fact at present is that no non-coding RNAs based therapeutic measures are put into clinical use. In addition, although our study has achieved progress in understanding the axis of miR-27a/USP46 in EC, the limitation here is we did not verify the expression of miR-27a and USP46 in EC tissues. Besides that, the role of miR-27a/USP46 axis on tumor progression was not investigated in vivo.
In summary, we confirmed that miR-27a promotes EC cell growth and migration through targeting the expression of USP46. The reports concluded that miR-27a/USP46 axis is the mechanism responsible for the malignancy progression of EC cells. Therefore, targeting miR-27a/USP46 axis could be a therapeutic approach for EC.
Disclosure of conflict of interest
None.
References
- 1.Brooks N, Pouniotis DS. Immunomodulation in endometrial cancer. Int J Gynecol Cancer. 2009;19:734–740. doi: 10.1111/IGC.0b013e3181a12f7f. [DOI] [PubMed] [Google Scholar]
- 2.Banno K, Nogami Y, Kisu I, Yanokura M, Umene K, Masuda K, Kobayashi Y, Yamagami W, Susumu N, Aoki D. Candidate biomarkers for genetic and clinicopathological diagnosis of endometrial cancer. Int J Mol Sci. 2013;14:12123–12137. doi: 10.3390/ijms140612123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran AQ, Gehrig P. Recent advances in endometrial cancer. F1000Res. 2017;6:81. doi: 10.12688/f1000research.10020.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Vasilatou D, Sioulas VD, Pappa V, Papageorgiou SG, Vlahos NF. The role of miRNAs in endometrial cancer. Epigenomics. 2015;7:951–959. doi: 10.2217/epi.15.41. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Cai Y, An R. miR‑215 promotes epithelial to mesenchymal transition and proliferation by regulating LEFTY2 in endometrial cancer. Int J Mol Med. 2018;42:1229–1236. doi: 10.3892/ijmm.2018.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Li C, Jiang Y, Wan Y, Zhou S, Cheng W. Tumor-suppressor role of miR-139-5p in endometrial cancer. Cancer Cell Int. 2018;18:51. doi: 10.1186/s12935-018-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu J, Zhang L, Li L, Su Y. miR-148b functions as a tumor suppressor by targeting endoplasmic reticulum metallo protease 1 in human endometrial cancer cells. Oncol Res. 2018;27:81–88. doi: 10.3727/096504018X15202988139874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Zhang LY, Chen Y, Jia J, Zhu X, He Y, Wu LM. MiR-27a promotes EMT in ovarian cancer through active Wnt/β-catenin signalling by targeting FOXO1. Cancer Biomark. 2019;24:31–42. doi: 10.3233/CBM-181229. [DOI] [PubMed] [Google Scholar]
- 10.Chae DK, Ban E, Yoo YS, Kim EE, Baik JH, Song EJ. MIR-27a regulates the TGF-β signaling pathway by targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog. 2017;56:1992–1998. doi: 10.1002/mc.22655. [DOI] [PubMed] [Google Scholar]
- 11.McClurg UL, Azizyan M, Dransfield DT, Namdev N, Chit NCTH, Nakjang S, Robson CN. The novel anti-androgen candidate galeterone targets deubiquitinating enzymes, USP12 and USP46, to control prostate cancer growth and survival. Oncotarget. 2018;9:24992–25007. doi: 10.18632/oncotarget.25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Stevens PD, Yang H, Gulhati P, Wang W, Evers BM, Gao T. The deubiquitination enzyme USP46 functions as a tumor suppressor by controlling PHLPP-dependent attenuation of Akt signaling in colon cancer. Oncogene. 2013;32:471–478. doi: 10.1038/onc.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Qian K, Wei X, Deng H, Zhao B, Chen Q, Zhang J, Liu H. miR‑27a promotes proliferation, migration, and invasion of colorectal cancer by targeting FAM172A and acts as a diagnostic and prognostic biomarker. Oncol Rep. 2017;37:3554–3564. doi: 10.3892/or.2017.5592. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Tong JL, Zhang CP, Xiao Q, Lin XL, Xiao XY. miR-27a induced by colon cancer cells in HLECs promotes lymphangiogenesis by targeting SMAD4. PLoS One. 2017;12:e0186718. doi: 10.1371/journal.pone.0186718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slaby O, Laga R, Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochem J. 2017;474:4219–4251. doi: 10.1042/BCJ20170079. [DOI] [PubMed] [Google Scholar]
- 16.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis. 2017;38:485–491. doi: 10.1093/carcin/bgx026. [DOI] [PMC free article] [PubMed] [Google Scholar]


