Fig. 3.
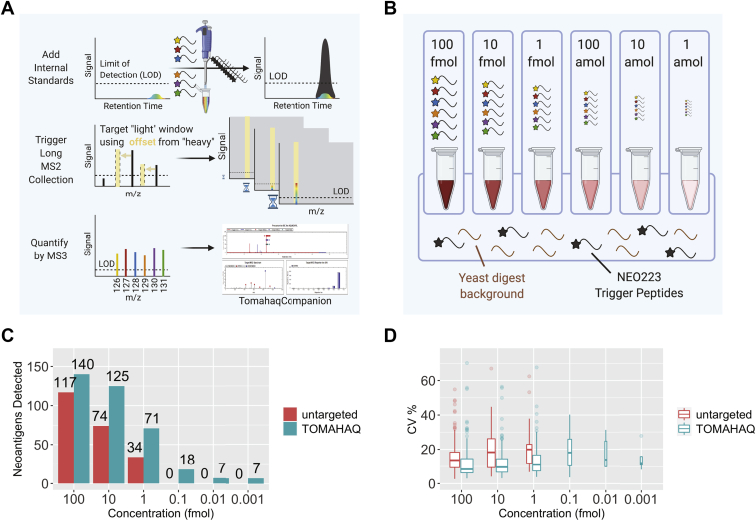
TOMAHAQ is more sensitive and quantitative than untargeted MS against a synthetic neoepitope mixture.A, scheme showing how TOMAHAQ uses the detection of added internal standard peptides to trigger detection of endogenous TMT-tagged targets. Internal standard triggers (black lines) are first added to the multiplexed mixture (multicolor). MS1 detection of the trigger peptide m/z leads to fragmentation, which, if the correct fragment ions are observed on the MS2 level, causes collection and fragmentation of the endogenous peptide via m/z offset followed by a long MS2 fragment collection time (900 ms) to build up signal. The most intense ions are then selected and sent to MS3 for quantification and demultiplexing over a very long collection time (2500 ms). B, scheme illustrating the 6-plex equimolar dilution series experimental setup where 100 fmol/μl per NEO223 peptide across each TMT-6plex channel was added to a constant background of 50 ng/ml yeast digest and 500 fmol/μl per NEO223 TMT-SH trigger peptide. This mixture was then tenfold serially diluted in constant background (yeast digest plus triggers) down to 1 amol/μl per NEO223 peptide across each TMT-6plex channel. C, comparison of total neoantigens detected with decreasing concentration of target peptides in constant background, between TOMAHAQ and an untargeted method. D, percent coefficient of variation (CV) for the experiment in C.