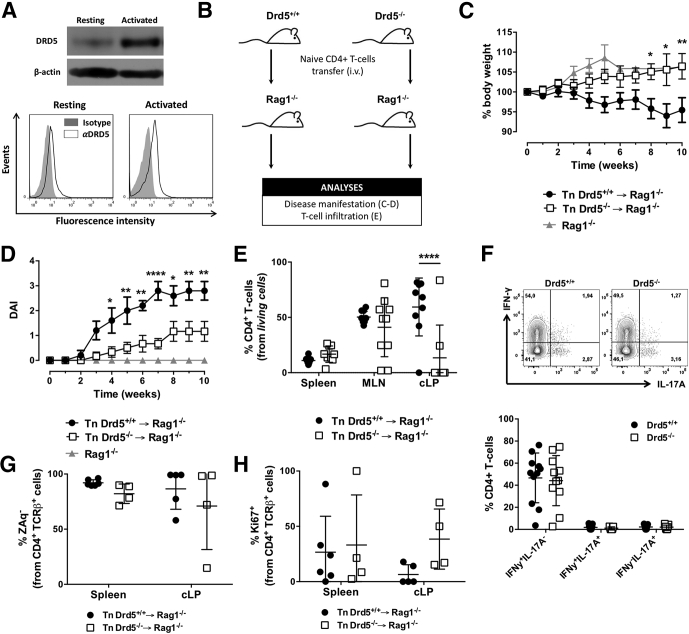
Figure 1.
Deficiency of DRD5 signaling in CD4+T cells dampens the development of inflammatory colitis and results in reduced frequency of CD4+T cells in the colonic lamina propria without affecting the acquisition of inflammatory phenotypes. (A) CD4+ T cells purified from wild-type mice were left unstimulated or activated with anti-CD3 and anti-CD28 mAbs for 24 hours. In the top panel, the expression of DRD5 was evaluated by Western blots. β-actin was used as a control. In the bottom panel, cells were immunostained with anti-DRD5 antibody (open histograms) or with irrelevant isotype matched control (filled histograms) and analyzed by flow cytometry. Representative results from 1 of 3 independent experiments are shown. (B–E) Naïve CD4+ T cells (CD3+CD4+CD45RBhigh; Tn) were isolated from the spleen of Drd5+/+ (black symbols) or Drd5–/– (white symbols) mice and intraperitoneally transferred into Rag1–/– mice (5 × 105 per mouse) and the extent of disease manifestation and CD4+ T cell infiltration in different tissues were determined. (B) Scheme illustrating the experimental design. (C, D) Body weight loss (represented as % respective to the initial body weight) and disease activity index (DAI) were monitored once a week throughout 10 weeks. Values represent mean ± SEM: (C) n = 22–27 or (D) n = 5–6 mice/group. (C, D) Nontransferred Rag1–/– mice (grey symbols) were used as a control group (n = 4). (E) Ten weeks after T cell transfer, mice were sacrificed and the frequency of CD4+ T cell was evaluated in secondary lymphoid organs (spleen and MLN) and in the cLP by flow cytometry. n = 8–10 mice/group. (F) Naïve CD4+ T cells (CD3+CD4+CD45RBhigh; Tn) were isolated from the spleen of Cd45.1+/+Drd5+/+ (black symbols) or Cd45.2+/+Drd5–/– (white symbols) mice, mixed in a 1:1 ratio and intraperitoneally injected (5 × 105 total cells per mouse) into Rag1–/– mice. Ten weeks later, mononuclear cells were isolated from the cLP, restimulated ex vivo and intracellular immunostaining of IFN-γ and IL-17 were analyzed by flow cytometry in the TCRβ+CD4+ population. Top panel shows representative contour plots of IFN-γ vs IL-17 from the CD45.1+ (Drd5+/+, left) and CD45.2+ (Drd5–/–, right) CD4+ T cells. Numbers indicate the percentage of cells in the corresponding quadrant. The bottom panel shows the quantification of the frequency of single producers IFN-γ+ or IL-17+ or double producers IFN-γ+ IL-17+ CD4+ T cells. n = 11 mice/group. (G, H) Mice were treated as indicated in panel B, and 12 weeks later, the extent of CD4+ T cell survival and proliferation were determined in the spleen and cLP as the percentage of (G) ZAq– cells and the frequency of (H) Ki67+ cells, respectively, on the CD4+ TCRβ+ gate by flow cytometry analysis. n = 4–6 mice/group. (E–H) Each symbol represents data obtained from an individual mouse. Mean ± SD are indicated. (C–H) ∗P < .05; ∗∗P < .001; ∗∗∗∗P < .0001 (Drd5+/+ vs Drd5–/–) by 2-way ANOVA followed by Sidak’s post hoc test.