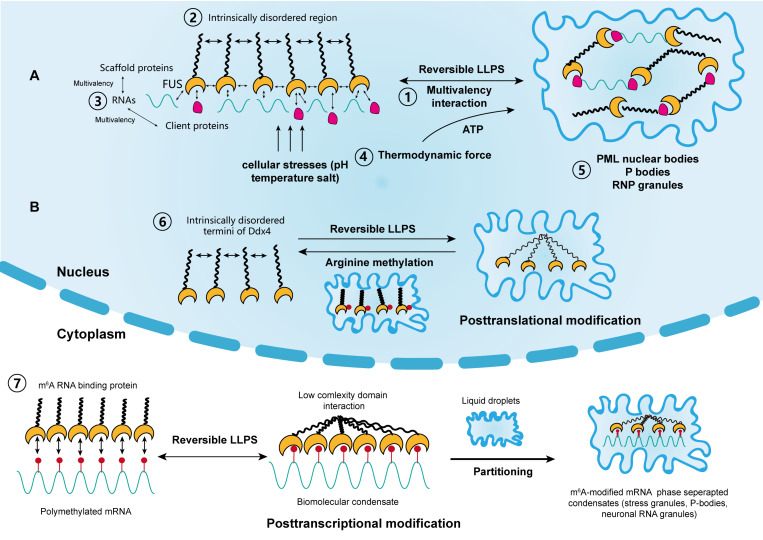
FIGURE 1.
The driving forces and regulation of liquid–liquid phase separation (LLPS) in cellular activity. (A) Scaffold proteins, such as FUS, concentrate low-valency client proteins through multivalent interactions, which is key for driving LLPS. The intrinsically disordered regions in some scaffolds also promote this process. Moreover, RNAs can further promote this process through interactions with RNA-binding regions. The thermodynamic force as well as cellular stressors, such as pH, temperature, and salt, may also support the reversible LLPS process. Classical nuclear structures formed by LLPS include PML bodies, P-bodies, and RNP granules. (B) LLPS is regulated by posttranslational and posttranscriptional modifications. Liquid condensates formed by Ddx4 are destabilized by its arginine methylation, whereas interactions between poly m6A methylated mRNAs and m6A binding proteins promote LLPS. Each step corresponds to its numbering.