| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac | Code/Name | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|---|---|---|
| N d | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐RNOTXBHXSA‐N |

|
Dd RU45199 | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1c[C@@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐BIXLXFTDSA‐N |

|
| Od Acrinathrin RU38702 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐WEQBUNFVSA‐N |

|
Cd RU 48238 | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐QTGMIWFCSA‐N |

|
| P d RU45120 ( 1S , cis, Z, αS) | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐BJRXWDRESA‐N |
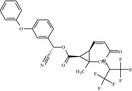
|
Bd R‐Acrinathrin RU 45120 (1R, cis, Z, αR) | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐UTFPFDPTSA‐N |

|
| Qd Z‐trans‐Acrinathrin RU39592 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐CEGVRPJUSA‐N |

|
A d | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1YLFSVIMMRPNPFK‐LVHZCJPFSA‐N |

|
| J e | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐WWLKQQBESA‐N |

|
He RU45198 | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐CEDMXCAPSA‐N |

|
| Ke E‐Acrinathrin RU39319 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐HIMIUADYSA‐N |

|
F e | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐KPJVQRDYSA‐N |

|
| L e | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐IJSXBTRHSA‐N |

|
Ge RU45168 | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐OCFKXNCMSA‐N |

|
| Me E‐trans‐Acrinatrhin RU39506 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐SFKSTPLFSA‐N |

|
Ee | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐SZSGGGEMSA‐N |

|
| 3‐PBA 3‐PBAcid | 3‐phenoxybenzoic acid O=C(O)c1cc(Oc2ccccc2)ccc1 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |

|
|||
| DPA‐A | (2Z)‐3‐(3‐{[2‐amino‐2‐oxo‐1‐(3‐phenoxyphenyl)ethoxy]carbonyl}‐2,2‐dimethylcyclopropyl)prop‐2‐enoic acid OC(=O)/C=C\C1C(C(=O)OC(c2cccc(Oc3ccccc3)c2)C(N)=O)C1(C)C OGXOFXISOFSZPL‐QXMHVHEDSA‐N |

|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Z‐isomer.
E‐isomer.
