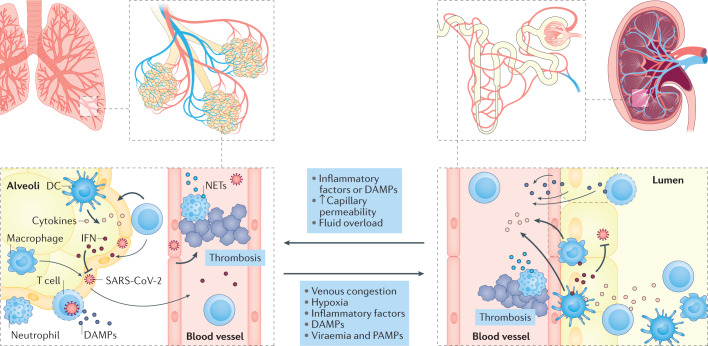
Fig. 1. Shared pathophysiology between lung and kidney injury in COVID-19.
Coronavirus disease 2019 (COVID-19)-associated acute respiratory distress syndrome involves regional inflammation with the recruitment of immune cells, including macrophages, effector T cells and polymorphonuclear neutrophils. Cytokines are released locally within the lung in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) and contribute to the further recruitment of inflammatory cells and tissue damage. Secretion of interferon (IFN) from immune cells contributes to viral clearance. Neutrophil extracellular traps (NETs), released by activated neutrophils, may also contribute to the local inflammatory response, pathogen clearance and thrombosis. Acute respiratory distress syndrome likely contributes to the development of acute kidney injury through systemic processes (for example, venous congestion and decreased cardiac output as a consequence of right-sided heart failure, high levels of intrathoracic pressure and hypoxia). Increased renal interstitial pressure due to tissue oedema is also likely to contribute to tubular injury. Release of DAMPs and PAMPs into the circulation contributes to regional inflammation within the kidney, the immune response and immune-mediated thrombosis. Direct infection of kidney cells has been observed in some patients and may also contribute to local inflammation and kidney damage. Conversely, acute kidney injury in other settings has been shown to contribute to promoting lung injury by stimulating regional inflammation, lung capillary permeability and fluid overload. DC, dendritic cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.