Abstract
Prompt implementation of appropriate targeted antibiotic therapy represents a valuable approach in improving clinical and ecological outcome in critically septic patients. This multidisciplinary opinion article focused at developing evidence-based algorithms for targeted antibiotic therapy of bloodstream (BSIs), complicated urinary tract (cUTIs), and complicated intrabdominal infections (cIAIs) caused by Enterobacterales. The aim was to provide a guidance for intensive care physicians either in appropriately placing novel antibiotics or in considering strategies for sparing the broadest-spectrum antibiotics. A multidisciplinary team of experts (one intensive care physician, one infectious disease consultant, one clinical microbiologist and one MD clinical pharmacologist), performed several rounds of assessment to reach agreement in developing six different algorithms according to the susceptibility pattern (one each for multi-susceptible, extended-spectrum beta-lactamase-producing, AmpC beta-lactamase-producing, Klebsiella pneumoniae carbapenemase (KPC)-producing, OXA-48-producing, and Metallo-beta-lactamase (MBL)-producing Enterobacterales). Whenever multiple therapeutic options were feasible, a hierarchical scale was established. Recommendations on antibiotic dosing optimization were also provided. In order to retrieve evidence-based support for the therapeutic choices proposed in the algorithms, a comprehensive literature search was performed by a researcher on PubMed-MEDLINE from inception until March 2021. Quality and strength of evidence was established according to a hierarchical scale of the study design. Only articles published in English were included. It is expected that these algorithms, by allowing prompt revision of antibiotic regimens whenever feasible, appropriate place in therapy of novel beta-lactams, implementation of strategies for sparing the broadest-spectrum antibiotics, and pharmacokinetic/pharmacodynamic optimization of antibiotic dosing regimens, may be helpful either in improving clinical outcome or in containing the spread of antimicrobial resistance.
Keywords: critically ill patients, targeted antibiotic therapy, antimicrobial stewardship, Enterobacterales, multidisciplinary taskforce, PK/PD dosing optimization
Introduction
Sepsis is a common occurrence in patients admitted to intensive care unit (ICU), accounting for high mortality and massive antibiotic consumption.1–3 Bloodstream infections (BSIs), complicated intra abdominal infections (cIAIs) and complicated urinary tract infections (cUTIs) are second only to pneumonia as sources of infections among ICU patients.4,5 Enterobacterales account for the most frequently isolated pathogens.5,6 Beta-lactams represent mainstay of treatment, and may have different roles according to the susceptibility pattern of clinical isolates. Previous antibiotic use, colonization by or ICU acquisition of MDR-Enterobacterales, prolonged hospitalisation, severity of acute illness are the major determinants of risk for developing infections caused by multidrug-resistant (MDR) Enterobacterales.7–9 Six different susceptibility patterns to beta-lactams may be identified among Enterobacterales: multi-susceptible, extended-spectrum beta-lactamase (ESBL)-producing, AmpC beta-lactamase (AmpC)-producing, Klebsiella pneumoniae carbapenemase (KPC)-producing, OXA-48-producing, and metallo-beta-lactamase (MBL)- producing Enterobacterales.7–9
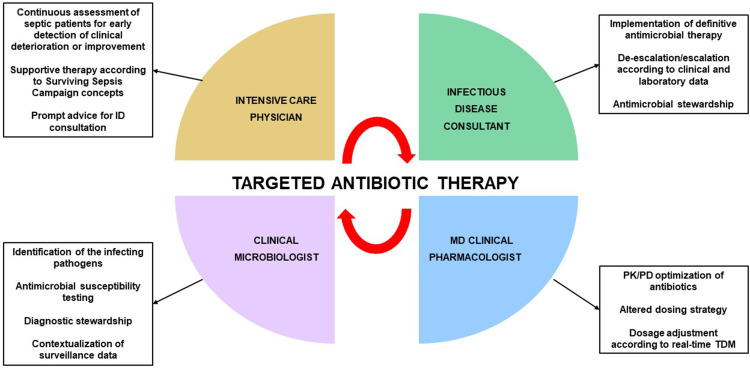
Early and appropriate antimicrobial treatment represents a cornerstone in the management of critically septic patient.10,11 The Surviving Sepsis Campaign guidelines recommend prompt implementation of targeted antibiotic therapy once that pathogen has been identified and antimicrobial susceptibility has been tested.12 A multidisciplinary team composed of the intensive care physician, the infectious disease consultant, the clinical microbiologist, and the MD clinical pharmacologist (Figure 1), could be helpful to pursuit this aim. Prompt implementation of appropriate definitive therapy according to the “antimicrobial puzzle” concepts13 could play a key role in improving clinical and ecological outcome in critical settings.14,15
Figure 1.
Features of multidisciplinary taskforce involved in implementation of targeted antimicrobial therapy in critically ill patients.
Abbreviations: ID, infectious disease; PCR, polymerase chain reaction; PK/PD; pharmacokinetic/pharmacodynamic; TDM, therapeutic drug monitoring.
This multidisciplinary opinion article aims to develop evidence-based algorithms for targeted antibiotic therapy of BSIs, cIAIs, and cUTIs caused by Enterobacterales in critically ill adult patients.
The aim was to provide a useful guidance for intensive care physicians either in appropriately placing novel antimicrobial agents in lack of definitive evidence or in considering antimicrobial stewardship strategies for sparing the broadest-spectrum antibiotics.
Materials and Methods
A multidisciplinary team composed by one intensive care physician (B.V.), one infectious disease consultant (P.V.), one clinical microbiologist (G.M.R.), and one MD clinical pharmacologist (F.P.) met virtually on several occasions to reach agreement in developing algorithms and specific recommendations for targeted antimicrobial therapy of BSIs, cIAIs, and cUTIs caused by Enterobacterales in ICU critically ill patients. The definitive agreement for each therapeutic algorithm was reached by the multidisciplinary team after thoroughly discussion based on specific long-standing experience and on the specific expertise of each single member. The rationale for considering common algorithms for these infection sites is based on the fact that cIAIs and cUTIs were investigated together in the last pivotal trials concerning novel antibiotics.16 Additionally, bacteraemic and non-bacteraemic cUTIs and cIAIs are commonly considered as relatively benign infection sources showing no high-inoculum effect, differently from that occurs in severe nosocomial pneumonia.17,18 Consequently, we believe that algorithms for targeted therapy of infection-related ventilator associated complications (IVACs) must be considered apart. Six different scenarios were structured according to the pattern of antibiotic susceptibility of the pathogens and/or of the genotype of resistance. Whenever multiple therapeutic options were feasible, a hierarchical scale was established. Recommendations on antibiotic dosing optimization were also provided.
Scientific evidence supporting the specific choices included in the algorithms was retrieved by means of a literature search conducted by a researcher (M.G.) on PubMed-MEDLINE (from inception until March 2021). Key terms for search included selected antibiotics, site of infections, and genotype of resistance and/or pattern of susceptibility of bacterial pathogens. Quality of evidence was established according to a hierarchical scale of the study design, as reported in the evidence pyramid:19 randomized controlled trials (RCTs); prospective observational studies; retrospective observational studies; case series; case reports; in vitro studies. International guidelines issued by the Infectious Disease Society of America and/or by the European Society of Clinical Microbiology and Infectious Diseases, systematic reviews and meta-analyses were also consulted. Consistence between retrieved studies was also considered, by assessing the concordance in clinical outcome of the included studies at each level of the evidence pyramid. Only articles published in English were included, and search was focused mainly on the last ten years in order to provide an up-to-date overview on the scientific evidence that may support the therapeutic algorithms.
Targeted Treatment of BSIs, cUTIs, and cIAIs Caused by Enterobacterales in Critically Ill Adult Patients
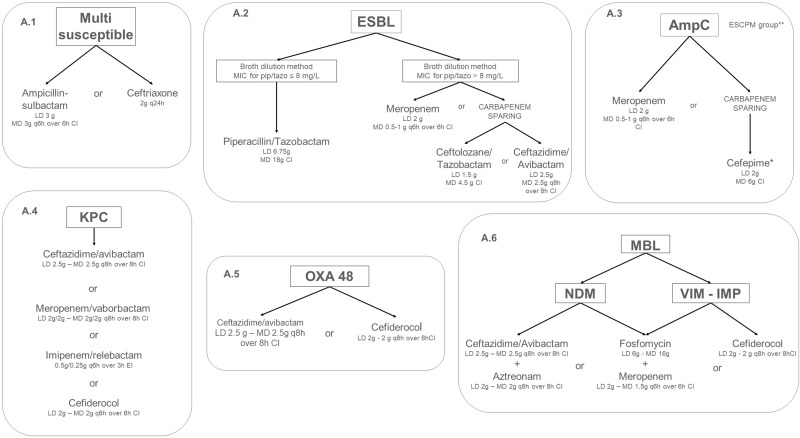
Six different algorithms for targeted treatment of BSIs, cUTIs, cIAIs are depicted in Figure 2, one each for infections caused by multi-susceptible, ESBL-, AmpC-, KPC-, OXA-48-, and MBL-producing Enterobacterales.
Figure 2.
Algorithms for targeted treatment of BSI, cUTI and cIAI, caused by Enterobacterales with different pattern of susceptibility in the ICU setting. *if MIC for cefepime ≤1 mg/L. ** ESCPM group includes: Enterobacter (E. cloacae complex, E. aerogenes), Serratia marcescens, Citrobacter freundii, Providencia stuartii, and Morganella morganii.
Abbreviations: CI, continuous infusion; MIC, minimum inhibitory concentration.
Multi-Susceptible Enterobacterales
Recommendations are depicted in Figure 2, panel A.1. Ampicillin-sulbactam [3g q6h over 6h by continuous infusion (CI) after 3g loading dose [LD]] or ceftriaxone (2g q24h) are recommended for BSIs, cUTIs, and cIAIs caused by multi-susceptible Enterobacterales. Evidences supporting these choices are summarized in Table 1. Both the European20 and the American21 guidelines recommended the use of ampicillin-sulbactam or ceftriaxone for the management of mild-to-moderate cIAIs/peritonitis. In regard to ampicillin-sulbactam, an RCT22 found no significant difference in clinical response rate between ampicillin-sulbactam and cefoxitin (86% vs 78%) in patients affected by cIAIs mainly due to Escherichia coli, although features concerning infection severity were not provided. A retrospective observational study found comparable clinical response rate between ampicillin-sulbactam and ticarcillin-clavulanate (84.3% vs 77.5%) among hospitalized patients with infections including cIAIs and cUTIs.23 Data on ICU admission and severity of infection were unavailable.
Table 1.
Summary of the Studies Investigating the Treatment of Full-Sensitive Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Ampicillin or Ceftriaxone
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | Isolates | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | |||||||||
| Eckmann et al, 201120 | Guidelines | Ampicillin-sulbactam 3 g q6h recommended for community-acquired localized peritonitis (Strength of recommendation: Grade A – Quality of evidence: Level I) | |||||||
| Walker et al, 199322 | Prospective randomized, double-blind, multicenter | 385 (194 AMP-SUL vs 191 cefoxitin) |
AMP-SUL 3 g q6h vs Cefoxitin 2 g q6h |
cIAI | E. coli most frequent | NA | Clinical response rate: 86% vs 78% |
Persistent or recurrent infections: 14% vs 22% |
AMP-SUL demonstrated no difference in efficacy when compared with cefoxitin in the treatment of serious bacterial cIAI |
| McKinnon et al, 199923 | Retrospective open-label, multicenter | 890 (664 AMP-SUL vs 226 TIC-CVL) |
AMP-SUL 1.5–3 g q6h vs TIC-CVL 3.1 g q6h |
258 SSTI 257 cIAI 200 respiratory 67 cUTI 67 gynaecologic 41 other |
E. coli and Klebsiella spp most frequent (frequencies not provided) | NA | Clinical response rate: 84.3% vs 77.5% Clinical response rate in cIAI: 88% vs 73% Clinical response rate in cUTI: 83.3% vs 84% |
NA | AMP-SUL had efficacy comparable with that of TIC-CVL in a variety of infections in hospitalized patients |
| Ceftriaxone | |||||||||
| Eckmann et al, 201120 | Guidelines | Ceftriaxone 2 g/day + Metronidazole 500 mg q8h recommended for community-acquired localized peritonitis (Strength of recommendation: Grade A – Quality of evidence: Level I) | |||||||
| Solomkin et al, 201021 | Guidelines | Ceftriaxone 2 g/day + Metronidazole 500 mg q8h recommended for mild-moderate community-acquired cIAI (Strength of recommendation: Grade A – Quality of evidence: Level I) | |||||||
| Wells et al, 200427 | Prospective randomized, double-blind, multicenter | 850 (377 ceftriaxone vs 473 ertapenem) |
Ceftriaxone 1 g/day vs Ertapenem 1 g/day |
cUTI | 64.7% E. coli 9.8% K. pneumoniae |
Severe disease 41.3% | Overall microbiological response rate: 91.1% vs 89.5% |
Bacterial recurrence: 7.6% vs 8.9% | No significant difference in efficacy between ceftriaxone and ertapenem in cUTI |
| Tomera et al, 200225 | Prospective randomized, double-blind, multicenter | 592 (294 ceftriaxone vs 298 ertapenem) |
Ceftriaxone 1 g/day vs Ertapenem 1 g/day |
cUTI 5.9% bacteraemic |
69.1% E. coli 13.1% K. pneumoniae |
Severe disease 42.2% | Overall microbiological response rate: 93% vs 91.8% |
Relapse and superinfection: 8.4% |
No significant difference in efficacy between ceftriaxone and ertapenem in cUTI |
| Rubinstein et al, 199524 | Prospective randomized controlled, multicenter | 580 (274 ceftriaxone + tobramycin vs 306 ceftazidime) |
Ceftriaxone 2 g/day + Tobramycin 3–5 mg/kg vs Ceftazidime 2 g q12h |
Serious hospital-acquired Gram-negative infections 297 pneumonia 184 sepsis 99 cUTI |
23.8% P. aeruginosa 20.6% Klebsiella spp 20.1 E. coli |
ICU admission 43.3% Mechanical ventilation 27.9% |
Clinical response rate: pneumonia 65% vs 73% sepsis 59% vs 73% cUTI 76% vs 80% |
Relapse and superinfection: 7.3% vs 4.6% (p=NS) |
No significant difference in efficacy between the two groups in severe hospital-acquired gram-negative infections |
| Park et al, 201228 | Prospective randomized, double-blind, multicenter | 271 (136 ceftriaxone vs 135 ertapenem) |
Ceftriaxone 1 g/day vs Ertapenem 2 g/day |
cUTI 33.6% bacteraemic |
85.3% E. coli 4.6% K. pneumoniae 4.4% ESBL+ |
NA | Overall microbiological response rate: 88.7% vs 87.9% |
NA | No significant difference in efficacy between ceftriaxone and ertapenem in cUTI |
| Jimenez-Cruz et al, 200226 | Prospective randomized, double-blind, multicenter | 258 (83 ceftriaxone vs 175 ertapenem) |
Ceftriaxone 1 g/day vs Ertapenem 1 g/day |
cUTI | 77.6% E. coli 6.6% K. pneumoniae |
Severe infection 39.1% | Overall microbiological response rate: 84.9% vs 85.6% |
Relapse and superinfection: 7.4% |
No significant difference in efficacy between ceftriaxone and ertapenem in cUTI |
Abbreviations: AMP-SUL, ampicillin-sulbactam; BSIs, bloodstream infections; cIAI, complicated intra abdominal infections; cUTI, complicated urinary tract infection; ESBL, extended-spectrum beta-lactamase; ICU, intensive care unit; NA, not available; NS, not significant; SSTI, skin and soft tissue infections; TIC-CVL, ticarcillin-clavulanate
In regard to ceftriaxone, an RCT comparing ceftriaxone plus tobramycin versus ceftazidime in the treatment of severe hospital-acquired Gram-negative infections (including BSIs and cUTIs) showed no significant difference in clinical response rate among patients who required ICU admission in 43.3% of cases.24 Several RCTs25–28 showed no significant difference in terms of microbiological response rate between ceftriaxone and ertapenem for the management of cUTIs caused mainly by E. coli and K. pneumoniae. Almost 40% of patients had severe infection and relapse rate was <10%. cUTI were bacteraemic in 5.9–33.6% of cases may provide, and this may support the efficacy of ceftriaxone in BSIs as well.
Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacterales
Recommentations are depicted in Figure 2, panel A.2. Piperacillin-tazobactam (18g CI after 6.75g LD) is recommended for the management of infections caused by ESBL-producing Enterobacterales with a piperacillin-tazobactam MIC ≤8 mg/L (according to the EUCAST breakpoint) tested by broth microdilution (the reference method for piperacillin-tazobactam susceptibility testing). Conversely, meropenem (0.5–1g q6h over 6h CI after 2g LD) should be preferred whenever piperacillin/tazobactam MIC is >8 mg/L. Ceftolozane-tazobactam (1.5g LD followed by 4.5g CI) and ceftazidime-avibactam (2.5g LD followed by 2.5g q8h over 8h CI) may represent alternative options when focusing at a “carbapenem-sparing” approach.29 In settings with high prevalence of carbapenem-resistant Enterobacterales (CRE), ceftazidime-avibactam should be reserved for CRE treatment to avoid epidemiological shift of carbapenemase producers to metallo-beta-lactamases (MBLs).29,30 Evidences supporting these choices are summarized in Table 2. Several studies compared piperacillin-tazobactam with carbapenems in the treatment of BSIs, cUTIs, and cIAIs caused by ESBL-producing Enterobacterales. Overall, an inconsistence emerged from the available evidence. The MERINO trial31 was the first large RCT that assessed the efficacy of a carbapenem-sparing strategy by comparing piperacillin-tazobactam vs meropenem in the treatment of ceftriaxone-resistant Escherichia coli or Klebsiella pneumoniae BSIs. Non-inferiority was not achieved in the piperacillin-tazobactam arm, as an overall 30-day all-cause mortality rate 3-fold higher than in the meropenem arm was observed (12.3% vs 3.7%, p=0.90). This arose concerns regarding the use of piperacillin-tazobactam as empirical or definitive therapy of ESBL-producing Enterobacterales BSIs.17 Indeed, it should be mentioned that a number of issues affected trial conclusions.17,32 The low mortality rate in the meropenem group was an unexpected finding; the primary source of BSI was imbalanced between arms (higher UTIs rate in the meropenem group); the number of neutropenic and immunocompromised patients was higher in the piperacillin-tazobactam group; sample size calculation was suboptimal; there was a high prevalence of blaOXA-1 genes (67%) strictly associated with high MICs for piperacillin-tazobactam (8–16 mg/L);33 pharmacokinetic/pharmacodynamic (PK/PD) properties of piperacillin-tazobactam were not maximized (administration by intermittent rather than by prolonged infusion). Conversely, a large body of evidence coming from well-design observational studies and systematic review34–46 showed no significant difference in efficacy and mortality rate between piperacillin-tazobactam and carbapenems among patients with ESBL-producing primary or secondary BSI in settings with ICU admission rate up to 40%. An RCT of piperacillin-tazobactam vs ertapenem found no significant difference in clinical cure and mortality rate among patients with bacteraemic cUTIs.47 Two studies showed a lower occurrence of MDR bacterial or fungal infections at 30-day from treatment with piperacillin-tazobactam compared to carbapenems (7.4% vs 24.6%; p=0.01),39 and a trend toward lower CRE isolation rate (2% vs 8%; p=0.09).46 Conversely, two other observational studies showed significantly lower mortality rate in patients treated with carbapenems compared to piperacillin-tazobactam.48,49 However, in one of this48 some limits must be recognized, as 61% of patients received lower-than-desirable dosage of piperacillin-tazobactam (3.375g q6h) by intermittent infusion, and 60% of Enterobacterales isolates had piperacillin-tazobactam MIC ≥8 mg/L. Overall, the available literature may provide support for considering piperacillin-tazobactam a valuable carbapenem-sparing agent in the management of ESBL-related infections when dealing with fully susceptible pathogens (MIC ≤8 mg/L). Notably, CI of high-dose piperacillin-tazobactam (18g) should be strongly recommended to achieve optimal PK/PD target in critically ill patients affected by ESBL-producing Enterobacterales infections, especially when dealing with isolates exhibiting high MIC values.50,51 Evidence supporting ceftolozane-tazobactam and/or ceftazidime-avibactam as carbapenem-sparing options for the treatment of for BSIs, cUTIs, and cIAIs caused by ESBL-producing Enterobacterales came from the non-inferiority showed vs carbapenems in pivotal Phase III trials.52–57 Indeed, it should be recognized that the overall proportion of ESBL-producing isolates was 100% only in one study that enrolled exclusively patients with documented ceftazidime-resistant infections,57 whereas it was <20% in all of the others.
Table 2.
Summary of the Studies Investigating the Treatment of Extended Spectrum Beta-Lactamase (ESBL)-Producing Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Carbapenems Compared to Piperacillin-Tazobactam or Novel Beta-Lactam/Beta-Lactamase Inhibitors (BL/BLIs)
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | ESBL-Producing Pathogens and Molecular Profile | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Piperacillin-tazobactam vs carbapenems | |||||||||
| Harris et al, 201831 | Randomized controlled, open-label, parallel-group, multicentric | 379 (188 PIP-TZB vs 191 MER) |
PIP-TZB 4.5 g q6h II vs MER 1 g q8h II |
All BSI 231 cUTI 62 cIAI 12 pneumonia 6 CR-BSI 5 SSTI 63 others |
Ceftriaxone-resistant Enterobacterales 327 E. coli 52 K. pneumoniae |
ICU admission 7.1% Immunocompromised 24% Neutropenia 6.6% Mean APACHE II score 17.9 vs 21 |
30-day mortality rate: 12.3% vs 3.7% (p=0.90 for non-inferiority) |
NA | Definitive treatment with PIP-TZB compared to MER did not result in non-inferior 30-day mortality rate |
| Seo et al, 201747 | Randomized controlled, multicenter | 66 (33 PIP-TZB vs 33 ERT) |
PIP-TZB 4.5 g q6h vs ERT 1 g/day |
All cUTI 16 secondary BSI |
100% E. coli MIC PIP-TZB 16 mg/L (72.7% of isolates) |
Septic shock 30.3% Mean APACHE II score 12.9 vs 16.6 |
28-day mortality rate: 6.1% vs 6.1% |
0% vs 0% | No difference between PIP-TZB and ERT in mortality and clinical cure rate |
| Rodriguez-Bano et al, 201235 | Post-hoc analysis of six prospective cohort | 204 Empiric: 35 PIP-TZB vs 31 carbapenems Definitive: 18 PIP-TZB vs 120 carbapenems |
PIP-TZB vs MER/IMI/ERT |
100% BSI 70% cUTI or biliary source |
100% E. coli | ICU admission 13% Severe sepsis or septic shock 23% Neutropenia 5.8% Immunocompromised 13.5% |
30-day mortality rate Empiric treatment: 9.7% vs 19.4% Definitive treatment: 9.3% vs 16.7% |
NA | PIP-TZB is a suitable alternative to carbapenems for treating patients with BSIs caused by ESBL-producing Enterobacterales |
| Gutierrez-Gutierrez et al, 201638 | Retrospective international cohort | 887 (Empiric: 123 PIP-TZB vs 195 carbapenems Definitive: 60 PIP-TZB vs 509 carbapenems) |
PIP-TZB 4.5 g q6-8h vs MER 1 g q8-12h or ERT 1 g/day or IMP 0.5 g q6-8h |
All BSIs 440 cUTI 120 biliary |
73% E. coli 19% K. pneumoniae 8% Other |
ICU admission 11.8% Severe sepsis or septic shock 37.7% Median Pitt score 1 |
30-day mortality rate Empiric treatment: 17.6% vs 20% Definitive treatment: 13.9% vs 9.8% |
NA | PIP-TZB, if active in vitro, appears to be as effective as carbapenems for empiric and target therapy of BSI due to ESLB-producing Enterobacterales regardless of the source and specific species |
| Gudiol et al, 201740 | Retrospective propensity matched cohort | 416 (Empiric: 44 PIP-TZB vs 126 carbapenems Definitive: 12 PIP-TZB vs 234 carbapenems) |
PIP-TZB vs MER (223) or IMI (110) or ERT (27) |
All BSIs 52.8% primary 18.1% CR-BSI 8.1% neutropenic colitis 6.9% cUTI |
74% E. coli 23% K. pneumoniae 1.5% K. oxytoca 1.5% E. cloacae |
ICU admission 20% Septic shock 24.3% Haematological neutropenic patients 100% |
30-day mortality rate Empiric treatment: 20.8% vs 13.4% (p=0.33) Definitive treatment: 5.8% vs 15.8% (p=0.99) |
Persistent BSI: 17.6% vs 4.7% (p=0.059) No difference in superinfection and colonization-infection by MDR isolates |
PIP-TZB could be carbapenem-sparing alternatives for the treatment of BSI due to ESBLs in haematological patients. |
| Nasir et al, 201945 | Retrospective cohort, monocentric | 263 (89 PIP-TZB vs 174 carbapenems) |
PIP-TZB vs carbapenems |
All BSIs 195 cUTI 68 cIAI 8 CR-BSI |
100% E. coli (ceftriaxone-resistant) | ICU admission 38% Septic shock 17% |
Overall mortality rate for definitive therapy: 13% vs 21% (p=NS) |
NA | PIP-TZB showed similar efficacy compared to carbapenems |
| Ko et al, 201842 | Retrospective cohort, multicentric | 224 (41 PIP-TZB vs 183 carbapenems) |
PIP-TZB vs carbapenems |
All BSIs 86 cUTI 65 cIAI 56 primary BSI 8 CR-BSI 17 other |
159 E. coli 73 K. pneumoniae |
ICU admission 34.4% | 30-day mortality rate: 6.3% vs 11.4% Empirical use of PTZ was not associated with 30-day all-cause mortality (HR 1.21, CI 0.37–4.00) |
NA | Appropriate non-carbapenems were not inferior to carbapenems as initial empirical therapy for ESBL-BSIs |
| Tamma et al, 201548 | Retrospective propensity score matched | 213 (103 PIP-TZB vs 110 carbapenems) |
PIP-TZB 3.375/4.5 g q6h II vs MER/IMI/ERT (MER 1–2 q8h II) |
100% BSI 97 CR-BSI 44 cUTI 36 cIAI 20 pneumonia 19 biliary |
68% K. pneumoniae 31% E. coli 1% P. mirabilis MIC PIP-TZB ≥8 mg/L 60% |
ICU admission 33.8% Neutropenia 15% Immunocompromised 58.7% |
14-day mortality rate: 17% vs 8% (p< 0.05) 30-day mortality rate: 26% vs 11% (p< 0.05) |
NA | The adjusted risk of death was 1.92 times higher for patients receiving empiric PIP-TZB compared with empiric carbapenem therapy (95% CI: 1.07–3.45) |
| Sharara et al, 202046 | Retrospective cohort, multicenter | 186 (45 PIP-TZB vs 141 carbapenems) |
PIP-TZB vs carbapenems |
100% cUTI All non-bacteraemic |
56% E. coli 30% K. Pneumoniae 10% P. mirabilis 4% K. oxytoca |
ICU admission 27% Septic shock 5.9% Immunocompromised 48% |
30-day mortality rate: 4% vs 7% |
30-day recurrent cUTI: 20% vs 25% (OR 0.75; CI 0.31–1.81) 30-day CRE isolation: 2% vs 8% (p=0.09) |
PIP-TZB may be a reasonable alternative to carbapenems for the management of ESBL-producing cUTI and may mitigate the risk of emergence of carbapenem-resistant organisms |
| Ng et al, 201639 | Retrospective cohort multicenter | 151 (94 PIP-TZB vs 57 carbapenems) |
PIP-TZB 4.5 g q6h II or q8h EI vs MER 1 g q8h or ERT 1 g/day or IMP 0.5 g q6h |
All BSIs 89 cUTI 14 biliary 13 pneumonia 8 cIAI 6 CR-BSI 18 other |
67% E. coli 33% K. pneumoniae |
ICU admission 8.6% Median Pitt score 1 |
30-day mortality rate: 30.9% vs 29.8% (p= NS) |
30-day relapsed BSI: 3.2% vs 15.8% (p=0.05) |
PIP-TZB was significantly associated with lower acquisition of MDR bacterial or fungal infections at 30-day (7.4% vs 24.6%; p=0.01) |
| Yoon et al, 201741 | Retrospective cohort, monocentric | 150 (68 PIP-TZB vs 82 ERT) |
PIP-TZB 4.5 g q8h vs ERT 1 g/day |
All cUTI 23 secondary BSI |
100% E. coli | ICU admission 24.7% Septic shock 16% Immunocompromised 14% |
In-hospital mortality rate: 4.4% vs 13.4% (p=0.059) |
NA | MIC> 4–8 mg/L for PIP-TZB was not significant associated with treatment failure. PIP-TZB could be an effective alternative to ERT for the treatment of cUTI caused by ESBL isolates |
| John et al, 201944 | Retrospective cohort, multicenter | 117 (66 PIP-TZB vs 51 carbapenems) |
PIP-TZB vs MER/ERT |
All BSIs 85 cUTI 22 cIAI 1 Pneumonia 9 unknown |
101 E. coli 16 K. pneumoniae |
ICU admission 38.5% Septic shock 17.1% Mechanical ventilation 2.6% Immunocompromised 12% |
In-hospital mortality: 3% vs 7.8% |
Relapse ESBL-BSI: 7.6% vs 7.8% |
Empiric PIP-TZB use and avoidance of empiric carbapenem therapy in the first 24 hours of infection can be considered until a microbiological diagnosis is confirmed. |
| Kang et al, 201236 | Retrospective case-control | 114 (36 PIP-TZB vs 78 carbapenems) |
PIP-TZB vs Carbapenems |
100% BSI | 68% E. coli 32% K. pneumoniae |
Haematological malignancies 22.8% | 30-day mortality rate 22.2% vs 26.9% |
NA | At propensity score analysis, empirical therapy with PIP-TZB was not associated with mortality (OR 0.63; CI 0.17–2.27) |
| Ofer-Friedman et al, 201549 | Retrospective cohort | 79 (10 PIP-TZB vs 69 carbapenems) |
PIP-TZB vs MER/IMI/DOR/ERT |
Non urinary BSIs 27 pneumonia 22 SSTI 13 biliary 7 cIAI 6 CR-BSI 4 unknown |
53% E. coli 28% K. pneumoniae 19% P. mirabilis |
Mean Pitt bacteraemia score 3.1 Immunocompromised 28% |
30-day mortality rate: 60% vs 34% (p=NS) 90-day mortality rate: 80% vs 48% (p=0.05) |
NA | In multivariate analysis, therapy with PIP-TZB was associated with increased 90-day mortality (adjusted odds ratio, 7.9, P=0.03). For ESBL BSIs of a non-urinary origin, carbapenems should be considered a superior treatment to BL/BLIs |
| Benanti et al, 201943 | Retrospective cohort, monocentric | 63 (21 PIP-TZB vs 42 carbapenems – 41 MER/1 ERT) |
PIP-TZB 4.5 g q6h vs MER 1 g q8h |
All BSIs 25 cIAI 7 CR-BSI 7 pneumonia 6 cUTI 6 SSTI 12 unknown |
100% E. coli | ICU admission 30.2% Haematological malignancies 100% Neutropenia 88.9% Median Pitt score 2 |
14-day mortality rate: 0% vs 19% (p=0.04) |
Persistent bacteraemia: 36% vs 5% (p=0.03) |
Empiric treatment PIP-TZB did not result in increased mortality compared to carbapenems. In multivariate logistic regression, empiric treatment with PIP-TZB was a significant predictor of persistent BSI (aOR, 27.1; CI 1.8–410.1) |
| Harris et al, 201537 | Retrospective observational cohort | 47 (24 PIP-TZB vs 23 carbapenem) |
PIP-TZB 4.5 g q6h vs MER 1 g q8h or IMI 0.5g q6h or ERT 1 g/day |
All BSIs 22 cUTI 4 biliary |
Cefotaxime non-susceptible BSIs 39 E. coli 8 K. pneumoniae |
ICU admission 14.9% Median Pitt score 1 Median APACHE II score 24 Immunocompromised 12.8% Neutropenia 2.1% |
30-day mortality rate: 8.3% vs 17.4% (p=NS) |
0% vs 2% | BL/BLIs appear to have a similar efficacy to carbapenems in the treatment of cefotaxime-resistant Enterobacterales BSIs. Directed therapy with a BL/BLI, when susceptibility is proven, may represent an appropriate carbapenem-sparing option. |
| Novel BL/BLIs (Carba-sparing) | |||||||||
| Solomkin et al, 201553 | Prospective, randomized, double-blind, multicentric | 993 (487 LOZ-TAZ vs 506 MER) |
LOZ-TAZ 1.5 g q8h + Metronidazole 0.5 g q8h vs MER 1 g q8h |
993 cIAI 20 secondary BSI |
Overall proportion of ESBL isolates: 7.2% 50 ESBL-producing 24 CTX-M-14/15 |
APACHE II score >15: 3.1% | Clinical cure in ESBL-isolates: 95.8% vs 88.5% Clinical cure in CTX-M-14/15 ESBLs: 100% vs 72.7% |
NA | Treatment with LOZ-TAZ plus metronidazole was noninferior to MER in adult patients with cIAI, including infections caused by MDR pathogens |
| Wagenlehner et al, 201552 | Randomized, double-blind, double-dummy, non-inferiority, multicenter | 800 (398 LOZ-TAZ vs 402 LEV) |
LOZ-TAZ 1.5 g q8h vs LEV 750 mg/day |
800 cUTI 62 secondary BSI |
72 E. coli 17 K. pneumoniae |
NA | Clinical cure: 76.9% vs 68.4% (percentage difference 8.5 CI 2.3–14.6) Microbiological eradication: 80.4% vs 72.1% (8.3 CI 2.4–14.1) |
NA | LOZ-TAZ superior to LEV in composite cure |
| Mazuski et al, 201655 | Prospective, randomized, multicenter, double-dummy, double-blind, comparative |
1066 (413 CAZ-AVI vs 410 MER in mMITT) |
CAZ-AVI 2.5 g q8h + Metronidazole 0.5 g q8h vs MER 1 g q8h |
1066 cIAI | Overall proportion of ceftazidime non-susceptible isolates: 13.5% (of which about 80% ESBL-producers) | APACHE II score >10: 15.3% | Clinical cure at TOC: 81.6% vs 85.1% (−3.5% CI −8.64–1.58%) Ceftazidime non-susceptible isolates (clinical response at TOC): 83% vs 85.9% (−3% CI −17.89–10.6%) |
NA | CAZ-AVI was highly effective for the empiric and definitive treatment of cIAI (including ceftazidime non-susceptible and ESBL-producers isolates), and may offer an alternative to carbapenems in this setting |
| Wagenlehner et al, 201654 | Randomized, multicenter, double-blind, double-dummy, parallel-group |
1033 (490 CAZ-AVI vs 492 DOR at TOC analysis) |
CAZ-AVI 2.5 g q8h vs DOR 0.5 g q8h |
1033 cUTI 71 secondary BSI |
Overall proportion of ESBL isolates: 147 (19.6%) | NA | Clinical resolution at day 5: 70.2% vs 66.2% (4% CI −2.39–10.42%) Microbiological eradication at TOC: 71.2% vs 64.5% (6.7% CI 0.3–13.12%) Ceftazidime non-susceptible isolates (microbiological response rate): 63.2% vs 58.2% (5% CI −10.87–20.5) |
NA | CAZ-AVI was highly effective for the empiric treatment of cUTI (including acute pyelonephritis), and may offer an alternative to carbapenems in this setting |
| Qin et al, 201756 | Prospective, randomized, multicenter, double-dummy, double-blind, comparative |
432 (215 CAZ-AVI vs 217 MER) |
CAZ-AVI 2.5 g q8h + Metronidazole 0.5 g q8h vs MER 1 g q8h |
432 cIAI 15 secondary BSI |
Overall proportion of ceftazidime non-susceptible isolates: 23.4% | APACHE II score >10: 6.7% | Clinical cure at TOC: 93.8% vs 94.2% (−0.2% CI −5.53–4.97%) Ceftazidime non-susceptible isolates (clinical response at TOC): 95.2% vs 96.0% (−0.8% CI −19.51–15.78%) |
NA | CAZ-AVI was highly effective for the empiric and definitive treatment of cIAI (including ceftazidime non-susceptible and ESBL-producers isolates) in Asian patients, and may offer an alternative to carbapenems in this setting |
| Carmeli et al, 201657 | Prospective, randomized, multicenter, double-dummy, double-blind, comparative |
333 (165 CAZ-AVI vs 168 BAT) |
CAZ-AVI 2.5 g q8h |
281 cUTI 21 cIAI |
Ceftazidime-resistant Enterobacterales and Pseudomonas aeruginosa | APACHE II score >10: 19% (only for cIAI) | Clinical cure at TOC: 91% vs 91% |
NA | CAZ-AVI was effective as a potential alternative to carbapenems in patients with ceftazidime-resistant Enterobacterales and P. aeruginosa. |
Abbreviations: BAT, best available therapy; BL/BLIs, beta-lactam/beta-lactamase inhibitors; BSIs, bloodstream infections; CAZ-AVI, ceftazidime-avibactam; CI, confidence interval; cIAI, complicated intra abdominal infections; CR-BSI, catheter-related bloodstream infections; CRE, carbapenem-resistant Enterobacterales; cUTI, complicated urinary tract infection; DOR, doripenem; EI, extended infusion; ERT, ertapenem; ESBL, extended-spectrum beta-lactamase; HR, hazard ratio; ICU, intensive care unit; IMI, imipenem; LEV, levofloxacin; LOZ-TAZ, ceftolozane-tazobactam; MDR, multidrug-resistant; MER, meropenem; MIC, minimum inhibitory concentration; mMITT, modified microbiological intention-to-treat; NA, not available; NS, not significant; OR, odds ratio; PIP-TZB, piperacillin-tazobactam; SSTI, skin and soft tissue infections; TOC, test of cure.
AmpC Beta-Lactamase-Producing Enterobacterales
Recommendations are depicted in Figure 2, panel A.3. Meropenem (0.5–1g q6h over 6h CI after 2g LD) is recommended as first-line treatment for BSIs, cUTIs, and cIAIs caused by AmpC-producing Enterobacterales. Cefepime (6g CI after 2g LD) may be a “carbapenem-sparing” alternative option if the MIC is ≤1 mg/L. AmpC beta-lactamases belong to the class C of the Ambler’s classification, and genes encoding for them are usually located in the chromosome of bacteria belonging to the so-called ESCPM group (namely Enterobacter cloacae complex, Enterobacter aerogenes, Serratia marcescens, Citrobacter freundii, Providencia stuartii, and Morganella morganii),58 but can also be carried on transferable plasmids and found in isolates of other species (eg, E. coli, K. pneumoniae, P. mirabilis). AmpC may hydrolyse all the penicillins, the 1st, 2nd and 3rd cephalosporins, and the monobactam aztreonam, but not the 4th generation cephalosporins and the carbapenems. Furthermore, beta-lactamase inhibitors (tazobactam, sulbactam, clavulanate) exhibit no activity against AmpC-producing isolates. Evidences coming from comparative studies between carbapenems and cefepime in the treatment of BSIs, cUTIs, and cIAIs caused by AmpC-producing Enterobacterales is provided in Table 3. Overall, a large body of evidence obtained from well-design prospective and retrospective observational studies and from systematic reviews showed no significant difference in terms of clinical cure and mortality rate between cefepime and carbapenems in settings characterized by ICU admission up to 60%.59–66 However, it should not be overlooked that cefepime was less effective against strains with an MIC ≥2 mg/L. One study showed significantly higher mortality rate in patients affected by cefepime-susceptible dose dependent (SDD) isolates (MIC 4–8 mg/L) who were treated with cefepime compared to those treated with carbapenems (71.4% vs 18.2%; p=0.045), even if a full-dose cefepime (6g/day) was administered only in 38.6% of cases.65 Likewise, higher rate of persistent bacteraemia was shown among patients affected with cefepime-SDD isolates who were treated with cefepime.62 However, only 16% of patients received full-dose cefepime.
Table 3.
Summary of the Studies Investigating the Treatment of AmpC-Producing Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Carbapenems and Cefepime
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | AmpC-Producing Pathogens and Molecular Profile | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Carbapenems vs Cefepime | |||||||||
| Harris et al, 201559 | Systematic review with meta-analysis | 11 studies (6 retrospective 5 prospective) |
Carbapenems vs BL/BLIs – cefepime - FQ | BSI |
Enterobacter spp Citrobacter spp Serratia spp Morganella spp |
8 studies including cefepime vs carbapenems. No significant difference in mortality rate was found for both empiric (OR 0.60; CI 0.17–2.20) or definitive therapy (OR 0.61; CI 0.27–1.38). | |||
| Tamma et al, 201361 | Prospective propensity score matched cohort | 78 (46 CEF vs 32 MER) |
Cefepime (1–2 g q8h) vs Meropenem (1–2 g q8h) Dose adjustment for renal impairment |
40 HAP 38 BSI 38 cIAI |
51 E. cloacae 31 E. aerogenes 13 S. marcescens 1 C. freundii |
ICU admission 42.7% vs 62.5% Mechanical ventilation 29.2% vs 37.5% Septic shock 22.9% vs 34.4% Immunocompromised 29.2% vs 50% |
No difference in 30-day mortality rate (OR 0.63 95% CI 0.23–2.11) No difference in hospital length of stay (OR 0.96 95% CI 0.79–1.26) |
Relapse 25% Resistance 1.6% (in cefepime group) |
Cefepime may be a reasonable option for the treatment of invasive infections due to AmpC β- lactamase–producing organisms |
| Tan et al, 202064 | Retrospective cohort study | 241 of which 189 with definitive therapy with CEF (N=57) or carbapenems (N=132) |
CEF (N=57) vs IMI (N=16) or MER (N=55) or ERT (N=61) |
55 cUTI 53 CR-BSI 46 cIAI 30 Pneumonia 13 SSTI 44 others All BSI |
140 Enterobacter spp 54 Serratia spp 40 M. morganii 5 C. freundii 2 Providencia spp |
ICU admission 21.6% | 30-day mortality rate: 5.3% CEF vs 18.9% carbapenems (p=0.02) At multivariate analysis carbapenems not associated with significant higher mortality compared to CEF (OR 2.25; CI 0.86–5.91) |
NA | Empirical PIP/TZB and definitive CEF were not associated with 30-day mortality compared to carbapenems |
| Cheng et al, 201766 | Retrospective matched case-control | 165 (88 PIP/TZB vs 77 CEF or MER) |
PIP-TZB vs CEF or MER |
33 cIAI 31 cUTI 28 HAP/VAP 22 CR-BSI 15 SSTI 36 others All BSI |
85 E. cloacae 43 S. marcescens 17 Citrobacter spp 15 E. aerogenes 2 S. liquefaciens 2 E. absuriae 1 E. cancerogenus |
ICU admission 40% RRT 17% Septic shock 24.2% Neutropenia 6.1% Immunocompromised 26.1% |
30-day mortality rate: 10% PIP/TZB vs 12% CEF or MER |
Persistent BSI 16% PIP/TZB vs 13% MER |
Piperacillin-tazobactam may be a suitable alternative for the treatment of BSIs with AmpC-producing Enterobacter, Serratia, and Citrobacter spp |
| Lee et al, 201565 | Retrospective cohort study | 144 (72 CEF vs 72 carbapenems) |
CEF (2–6 g/day) vs IMI (0.5 g q6h) or MER (1 g q8h) or ERT (1 g/day) |
53 CR-BSI 45 primary BSI 13 HAP 11 cUTI 11 cIAI 8 SSTI All BSI |
144 E. cloacae | Pitt score ≥ 4 38.9% Neutropenia 9% |
30-day mortality rate: 26.4% CEF vs 22.6% carbapenems Higher mortality rate with CEF vs carbapenems in CEF-SDD isolates (71.4% vs 18.2%; p=0.045) |
NA | CEF-SDD isolates (MIC 4–8 mg/L) independently associated with 30-day mortality at multivariate analysis |
| Siedner et al, 201462 | Retrospective cohort study | 271 of which 52 (36 CEF monotherapy vs 16 carbapenem monotherapy) |
Cefepime (<2 – 6 g/day) |
All BSI 76% primary BSI 7% cUTI 6% pneumonia 4% cIAI 4% CR-BSI |
271 Enterobacter spp | ICU admission 22% Neutropenia 19% Pitt score ≥5 7% Solid organ transplant 6% Haematopoietic stem cell transplant 6% |
No difference in-hospital mortality rate 17% CEF vs 26% carbapenem |
Persistent bacteraemia: 25% carbapenem vs 0% CEF (p=0.002) in monotherapy |
In patients who received cefepime with evaluable MIC results, only 2 of 74 (3%) patients with an isolate with a cefepime MIC of ≤2 μg/mL had persistent bacteraemia within 24 hours vs 6 of 23 (26%) patients with an MIC ≥ 4 μg/mL (P < 0.001) |
| Blanchette et al, 201460 | Retrospective matched case-control | 48 (32 CEF vs 16 ERT) |
Cefepime vs Ertapenem |
15 cUTI 10 BSI 9 SSTI 9 HAP 6 cIAI |
32 Enterobacter spp 9 Citrobacter spp 7 Serratia spp |
ICU admission 18.8% Immunocompromised 20.8% Median APACHE II score 11 vs 13.5 |
Clinical success: 88% CEF vs 69% ERT (p=0.138) |
Resistance: 25% ERT vs 17% CEF |
Cefepime may be a reasonable option for the treatment of invasive infections due to AmpC β- lactamase–producing organisms |
| Hilty et al, 201363 | Retrospective cohort study | 43 | Cefepime (2 g/day – 2 g q8h) Meropenem (1 g/day – 2 g q8h) Piperacillin-TZB Ceftriaxone |
51 primary BSI 6 cUTI 3 CR-BSI 67.4% Hospital-acquired |
57 E. cloacae | ICU admission 11.6% Septic shock 2.3% Mechanical ventilation 25.6% Immunocompromised 41.9% Neutropenia 30.2% |
Clinical cure rate: 88.9% CEF vs 92.3% carbapenems |
NA | Cefepime represents a safe therapeutic option and an alternative to carbapenems to treat BSIs due to Ecl when the prevalence of ESBL-producers is low. |
Abbreviations: BSIs, bloodstream infections; CEF, cefepime; cIAI, complicated intra abdominal infections; CR-BSI, catheter-related bloodstream infections; cUTI, complicated urinary tract infection; ERT, ertapenem; FQ, fluoroquinolones; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IMI, imipenem; MER, meropenem; MIC, minimum inhibitory concentration; NA, not available; PIP-TZB, piperacillin-tazobactam; RRT, renal replacement therapy; SDD, susceptible dose dependent; SSTI, skin and soft tissue infections.
Klebsiella Pneumoniae Carbapenemase (KPC)-Producing Enterobacterales
Recommentations are depicted in Figure 2, panel A.4. Ceftazidime-avibactam (2.5g LD followed by 2.5g q8h over 8h CI) is recommended as first-line therapy for the management of BSIs, cIAIs, and cUTIs caused by KPC-producing Enterobacterales. Meropenem-vaborbactam (2g/2g q8h over 8h CI after 2g/2g LD), imipenem-relebactam (0.5g/0.25g q6h over 3h), and cefiderocol (2g LD followed by 2g q8h over 8h CI) could be alternative options, and are listed in a hierarchical scale. A summary of scientific evidences is provided in Table 4. One prospective and eight retrospective observational studies support the role of ceftazidime-avibactam in the management of KPC-producing BSIs, cIAIs, and cUTIs in settings with an ICU admission up to 60%.67–75 Van Duin et al assessed prospectively 137 carbapenemase-producing Enterobacterales (CPE) infections (38 treated with ceftazidime-avibactam vs 99 with colistin-based regimens).67 Ceftazidime-avibactam showed a better adjusted probability of favourable outcome (64%; p=0.0012), and a 3.5-fold lower all-cause mortality rate than colistin-based regimens (8% vs 33%; p=0.001). Caston et al analysed retrospectively 31 CPE infections among hematologic patients (8 treated with CAZ-AVI vs 23 treated with other antibiotic combinations, mainly carbapenems and aminoglycosides).68 Patients treated with ceftazidime-avibactam showed significantly higher clinical cure rate (85.7% vs 34.8%; p=0.03). Tumbarello et al analysed 208 patients with KPC-producing Klebsiella pneumoniae BSIs (104 treated with ceftazidime-avibactam as second line therapy vs 104 treated with different rescue mono- or combo-treatments).69 Patients treated with ceftazidime-avibactam showed significantly lower 30-day mortality rate (36.5% vs 55.8%; p=0.005), and ceftazidime-avibactam was the only independent predictor of survival at multivariate analysis. Shields et al analysed 109 CPE infections, 13 of whom treated with ceftazidime-avibactam and the other 96 with other antimicrobials (mainly colistin, aminoglycosides and carbapenems).70 Patients receiving ceftazidime-avibactam showed significantly lower 30-day mortality rate (8% vs 31.3%; p=0.01) and higher clinical success rate (85% vs 40.6%; p=0.006). Very recently, Tumbarello et al analysed 577 patients with KPC-producing Klebsiella pneumoniae infections (67.8% with BSIs) treated with ceftazidime-avibactam.75 No difference in mortality rate was found between ceftazidime-avibactam monotherapy vs combination therapy (26.1% vs 25.0%; p=0.79). Notably, ceftazidime-avibactam prolonged infusion resulted protective against mortality at multivariate analysis (p=0.006). In regard to meropenem-vaborbactam, it should be mentioned that vaborbactam was specifically developed to restore the activity of meropenem against KPCs.76 A phase III RCT (TANGO II) assessed 47 patients affected by KPC-producing Enterobacterales infections, 32 of whom were treated with meropenem-vaborbactam and the other 15 with best-available therapy (including mono/combination therapy with colistin, carbapenems, aminoglycosides, tigecycline, or ceftazidime-avibactam alone). Meropenem-vaborbactam showed better clinical cure rate (65.6% vs 33.3%; p=0.03) and a trend toward lower mortality rate (15.6% vs 33.3%; p=0.20) compared to best available therapy.77 However, it should be recognized that patients enrolled in this RCT required ICU admission only in 15.6% of cases. More attractive evidence for meropenem-vaborbactam as targeted therapy for KPC-producing Enterobacterales infections in critically ill patients came from observational studies, in which ICU admission ranged from 65.4% to 70%.78–80 Clinical cure rate ranged 65–70%, and mortality rate 10–22.5%. Relapse rate of CPE infections ranged 11.5–15%, and in up to 5% of patients was reported resistance development to meropenem-vaborbactam. One retrospective study78 reported no significant difference between 26 patients receiving meropenem-vaborbactam and 105 receiving ceftazidime-avibactam in terms of clinical cure rate (69.2 vs 61.9%) and mortality rate (11.5 vs 19.1%). In regard to imipenem-relebactam, it’s worth mentioning that relebactam was combined to imipenem-cilastatin in order to restore activity against carbapenemase producing Enterobacterales and Pseudomonas aeruginosa.76 In a phase III RCT of patients with severe Gram-negative infections, imipenem-relebactam demonstrated significantly better clinical cure rate compared to imipenem plus colistin (71.4% vs 40%; p<0.05).81 However, infection by KPC-producing Enterobacterales was documented in only 4 out of the 21 patients enrolled in the imipenem-relebactam group. In regard to cefiderocol, in a phase III RCT 150 patients affected by carbapenem-resistant Gram-negative infections were randomized to cefiderocol (n=101) or best available therapy (including combination of aminoglycoside, carbapenems, colistin, fosfomycin or tigecycline) (n=49).82 Clinical and microbiological cure rates between groups did not significantly differ. However, the number of documented KPC-producing Enterobacterales infections was quite limited.
Table 4.
Summary of the Studies Investigating the Treatment of Klebsiella pneumoniae Carbapenemase (KPC)-Producing Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Ceftazidime-Avibactam, Meropenem-Vaborbactam, Imipenem-Relebactam and Cefiderocol
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | CPE-producing Pathogens and Molecular Profile | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime-avibactam | |||||||||
| van Duin et al, 201867 | Prospective observational multicentric, comparative |
137 (38 CAZ-AVI vs 99 colisitn-based treatment) |
CAZ-AVI 100% target therapy |
16 primary BSIs 9 HAP 6 cUTIs 6 SSTIs 2 others |
37 K. pneumoniae 1 Enterobacter spp All KPC-2 or −3 |
ICU admission 53% Pitt score > 4 18% Immunosuppressed 29% |
Mortality rate at 30-day: 8% vs 33% (p=0.001) |
NA | 64% of probability of better outcome with CAZ-AVI compared to colistin at DOOR analysis |
| Tumbarello et al, 202175 | Retrospective observational, non-comparative | 577 | CAZ-AVI 2 g/0.5 g q8h 100% target therapy Prolonged infusion 42.6% Monotherapy 28.6% |
391 BSI 71 cUTI 59 HAP 35 cIAI 21 others |
All KPC-producing K. pneumoniae | ICU admission 23.7% Mechanical ventilation 28.1% Neutropenia 3.8% Transplant recipient 14.9% Immunocompromised 7.8% |
Overall mortality rate at 30-day: 25.3% |
Relapse 10.9% Resistance development 3.5% |
Prolonged infusion of CAZ-AVI was negatively associated with mortality at multivariate analysis. No difference in mortality rate between monotherapy and combination therapy (26.1% vs 25.0%; p=0.79) |
| Tumbarello et al, 201969 | Retrospective observational multicentric, comparative | 138 treated with CAZ-AVI Case-control matching between 104 BSIs treated with CAZ-AVI and 104 BSI patients treated with other treatments* |
CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
104 BSI 13 HAP 12 cIAI 6 cUTI 3 others |
138 K. pneumoniae 138 KPC (93.5% meropenem MIC ≥16 mg/L) |
ICU admission 33.3% Mechanical ventilation 31.2% Septic shock 31.2% Median Pitt index 4 Neutropenia 10.9% |
Overall mortality rate at 30-day: 34.1% Mortality rate at 30-day (only BSIs) 36.5% vs 55.8% (p=0.005) |
Relapse 8.7% Resistance development 2.2% |
At multivariate analysis CAZ-AVI was the only variable independently associated with survival. |
| Shields et al, 201770 | Retrospective observational, comparative | 109 (13 CAZ-AVI vs 96 other treatments*) |
CAZ-AVI 100% target therapy |
3 primary BSIs 10 secondary BSIs (5 cUTI; 3 HAP; 2 cIAI) |
13 K. pneumoniae 9 KPC-2 4 KPC-3 (CAZ-AVI MIC 0.25–2 mg/L) |
ICU admission 46% RRT 15% Median Pitt score 4 Median APACHE II score 20 Immunocompromised 38% Solid organ transplant recipient 23% |
Mortality rate at 30-day: 8% vs 31.3% (p=0.01) Mortality rate at 90-day: 8% vs 44.8% Clinical cure rate at 14-day: 85% vs 40.6% (p=0.006) |
Relapse 15% | At multivariate analysis CAZ-AVI was an independent predictor of clinical success. |
| Shields et al, 201874 | Retrospective observational, non-comparative | 77 | CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
34 HAP 20 primary BSI 8 cUTI 7 cIAI 6 SSTI 1 mediastinitis 1 meningitis |
60 K. pneumoniae 9 E. coli 5 E. cloacae 1 E. aerogenes 1 K. oxytoca 1 Serratia marcescens 58 KPC |
Median SOFA 5 Median SAPS II 41 CRRT 21% Transplant recipient 26% |
Overall mortality rate at 30-day: 19% Overall mortality rate at 90-day: 31% Overall clinical cure rate: 55% |
Relapse 32% Resistance development 10% |
Pneumonia was an independent predictor of clinical and microbiological failure at multivariate analysis. RRT was an independent predictor of clinical failure and resistance development at multivariate analysis. |
| King et al, 201772 | Retrospective observational multicentric, non-comparative | 60 | CAZ-AVI 100% target therapy Dosage according to manufacturer’s instruction |
23 primary BSI 17 cUTI 16 HAP 8 SSTI 4 cIAI 2 osteomyelitis |
50 K. pneumoniae 5 E. coli 4 Enterobacter spp 1 Providencia stuartii 1 Serratia marcescens 1 K. oxytoca |
ICU admission 59% Mechanical ventilation 38% Septic shock 21% RRT 23% Median Pitt score 2 Solid organ transplant recipient 25% |
Overall in-hospital mortality rate: 32% Overall clinical cure rate: 65% |
NA | Patients who required a renal adjustment of CAZ-AVI trended toward high in-hospital mortality (42% versus 19% without renal adjustment, p= 0.0567). |
| Temkin et al, 201771 | Retrospective observational multicentric, non-comparative | 38 | CAZ-AVI 100% target therapy |
15 cIAI 7 HAP 4 SSTI 3 cUTI 7 primary BSI or CR-BSI 2 endocarditis 3 osteomyelitis 2 surgical site infection 3 Others |
34 K. pneumoniae 1 K. oxytoca 1 E. coli 2 P. aeruginosa 23 KPC 13 OXA-48 |
Septic shock 44.7% Mechanical ventilation 36.8% Immunosuppression 23% |
Overall mortality rate: 39.5% Overall clinical/microbiological cure rate: 73.7% |
5.3% relapse | |
| Shields et al, 201673 | Retrospective observational, non-comparative | 37 | CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
13 HAP 10 primary BSI 4 cIAI 4 SSTI 4 cUTI 1 mediastinitis 1 meningitis |
31 K. pneumoniae 3 E. coli 2 E. cloacae 1 E. aerogenes 16 KPC-3 13 KPC-2 7 CTX-M 4 OXA-1-like 1 ESBL 1 AmpC |
Mean SOFA 5 Mean SAPS-II 34 CRRT 16.2% Transplant recipient 30% |
Overall mortality rate at 30-day: 24% Overall clinical cure rate: 59% |
Relapse 27% Resistance development 8% |
Success rates were lower for patients who required CRRT: 17% vs 68%; p= 0.03. |
| Caston et al, 201768 | Retrospective observational multicentric, comparative | 31 (8 CAZ-AVI vs 23 other treatments*) |
CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
2 HAP 2 BSI 1 CR-BSI 1 cIAI 1 SSTI 1 other |
6 K. pneumoniae 1 E. coli 1 K. oxytoca 5 OXA-48 3 KPC |
ICU admission 16.7% Septic shock 37.5% Renal failure 25.0% Median Pitt index 3 Neutropenia 62.5% |
Clinical cure at-14 day: 85.7% vs 34.8% (p=0.03) Mortality rate at 30-day: 25.0% vs 52.2% (p=0.24) |
NA | CAZ-AVI associated with higher clinical cure rate compared to combination of other treatments. Trend to lower mortality rate with CAZ-AVI. |
| Meropenem-vaborbactam | |||||||||
| Wunderink et al, 201877 | Phase 3, randomized, prospective, multicenter, open-label |
47 (32 MER-VAB vs 15 BAT) |
MER-VAB 2 g/2 g q8h (3h-infusion) 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
14 primary BSI 12 cUTI/AP 4 HAP/VAP 2 cIAI |
29 K. pneumoniae 3 E. coli 1 E. cloacae 1 S. marcescens |
ICU admission 15.6% Immunocompromised 34.4% |
Clinical cure rate at the end of treatment: 65.6% vs 33.3% (p=0.03) Clinical cure rate at test of cure: 59.4% vs 26.7% (p=0.02) Mortality rate at 28-day: 15.6% vs 33.3% |
NA | Monotherapy with MER-VAB for CRE infection was associated with increased clinical cure, decreased mortality, and reduced nephrotoxicity compared with BAT |
| Ackley et al, 202078 | Retrospective observational cohort, multicenter, comparative | 131 (26 MER-VAB vs 105 CAZ-AVI) |
MER-VAB 2 g/2 g q8h 100% target therapy |
12 HAP/VAP (2) 8 cIAI (3) 3 SSTI (1) 1 primary BSI 1 cUTI (1) 1 other (1) () number of secondary BSIs |
15 Klebsiella spp 8 Enterobacter spp 3 E. coli 2 Citrobacter spp 1 Serratia spp |
ICU admission 65.4% Median APACHE II score 27 RRT 4.8% Immunocompromised 15.4% |
Overall clinical success: 69.2% vs 61.9% Mortality rate at 30-day: 11.5% vs 19.1% |
Relapse 11.5% No resistance development |
Similar rates of clinical success between MER-VAB and CAZ-AVI in KPC-producing CRE infections |
| Alosaimy et al, 202079 | Retrospective observational, multicenter, non-comparative | 40 | MER-VAB 2 g/2 g q8h 100% target therapy |
13 HAP/VAP 11 BSI 8 cUTI 5 cIAI 5 SSTI |
21 K. pneumoniae 9 E. cloacae 6 E. coli 3 B. cepacian 2 P. aeruginosa 1 A. baumannii 1 M. morganii 1 P. mirabilis 1 S. marcescens |
ICU admission 70% Median APACHE II score 17 |
Overall clinical success: 70% Mortality rate at 90-day: 22.5% |
Relapse 12.5% | |
| Shields et al, 202080 | Prospective observational, non-comapartive | 20 | MER-VAB 2 g/2 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
8 BSI 6 HAP/VAP 2 SSTI 2 UTRI 1 cUTI 1 cIAI |
14 K. pneumoniae 2 K. oxytoca 2 E. coli 1 E. cloacae 1 C. freundii 10 KPC-3 7 KPC-2 1 KPC-31 |
ICU admission 70% RRT 35% Median SOFA 5 Median APACHE II score 20 |
Clinical success at 30-day: 65% Mortality rate at 30-day: 10% |
Relapse 15% Resistance development 5% |
|
| Imipenem-relebactam | |||||||||
| Motsch et al, 201981 | Phase 3, randomized, prospective, multicenter, open-label |
31 (21 IMI-REL vs 10 IMI + COL) |
IMP-REL 0.5 g/0.25 g q6h 100% target therapy Dose adjustment according to renal function |
11 cUTI 8 HAP/VAP 2 cIAI |
16 P. aeruginosa 3 K. pneumoniae 1 E. cloacae 1 C. freundii 4 KPC |
APACHE II score > 15: 33.3% |
Overall response rate: 71.4% vs 70% Clinical response at 28-day: 71.4% vs 40% (p< 0.05) Mortality rate at 28-day: 9.5% vs 30.0% |
Relapse 9.5% | IMI-REL as a suitable treatment option for serious gram-negative infections, including CRE in high-risk patients. IMI/REL had comparable efficacy but significantly less nephrotoxicity and other AEs compared to COL. |
| Cefiderocol | |||||||||
| Bassetti et al, 202082 | Phase 3, randomized, prospective, multicenter, open-label |
150 (101 cefiderocol vs 49 BAT) |
Cefiderocol 2 g q8h (3h-infusion) 100% target therapy Dose adjustment according to renal function |
45 HAP/VAP 30 BSI 26 cUTI |
37 A. baumannii 27 K. pneumoniae 12 P. aeruginosa 5 S. maltophilia 2 E. coli 2 E. cloacae 2 A. nosocomialis |
ICU admission 56% Septic shock 19% Mechanical ventilation 50% Immunocompromised 27% Mean SOFA score 5.1 |
Mortality rate at 14-day: 19% vs 12% Mortality rate at 28-day: 25% vs 18% Overall clinical cure at the end of treatment: 66% vs 58% Overall microbiological cure at the end of treatment: 48% vs 26% |
A numerically higher proportion of patients with CRE infections achieved a clinical cure in the cefiderocol group than in the BAT group (66% vs 45%) | |
Abbreviations: AP, acute pyelonephritis; BAT, best available therapy; BSIs, bloodstream infections; CAZ-AVI, ceftazidime-avibactam; cIAI, complicated intra abdominal infections; COL, colistin; CR-BSI, catheter-related bloodstream infections; CRE, carbapenem-resistant Enterobacterales; CRRT, continuous renal replacement therapy; cUTI, complicated urinary tract infection; DOOR, desirability of outcome ranking; ICU, intensive care unit; IMI, imipenem; IMI-REL, imipenem-relebactam; KPC, Klebsiella pneumoniae-producing carbapenemase; MER-VAB, meropenem-vaborbactam; MIC, minimum inhibitory concentration; NA, not available; RRT, renal replacement therapy; SOFA, sequential organ failure assessment; SSTI, skin and soft tissue infections; URTI, upper respiratory tract infections; VAP, ventilator-associated pneumonia.
OXA-48-Producing Enterobacterales
Recommendations are depicted in Figure 2, panel A.5. Ceftazidime-avibactam (2.5g LD followed by 2.5g q8h over 8h CI) is recommended as first-line therapy for the management of BSIs, cIAIs, and cUTIs caused by OXA-48 and OXA-48-like-producing Enterobacterales. (avibactam inhibits OXA-48, and ceftazidime is stable to this enzyme).83 Cefiderocol (2g LD followed by 2g q8h over 8h CI) could be an alternative option. A summary of the studies evaluating the efficacy of ceftazidime-avibactam and cefiderocol in this setting is provided in Table 5. Alraddadi et al84 compared retrospectively 10 patients treated with ceftazidime-avibactam with 28 treated with other mono- or combo-therapy (colistin, carbapenems, aminoglycosides, tigecycline, quinolone, cotrimoxazole, and aztreonam) for the management of CPE. After restricting analysis to OXA-48 infections, no difference in clinical cure (75% vs 40%; p=0.21) and in mortality rate (37.5% vs 50%; p=0.69) were reported. In two observational studies concerning the treatment with ceftazidime-avibactam of infections caused by OXA-48-producing Enterobacterales,85,86 the clinical cure rate and mortality rate were similar to those found in other studies where it was used for the treatment of KPC infections [59–62]. Conversely, in one retrospective study assessing ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms,71 among the 13 patients who were affected by OXA-48 infections a trend toward lower microbiological cure (25% vs 75%; p=0.07) and survival to hospital discharge (22.7% vs 77.3%; p=0.07) compared to the 23 who had KPC infections was found. In regard to cefiderocol, there is only one case87 that reported its use for the management of a secondary BSI caused by carbapenem-resistant K. pneumoniae co-producing OXA-48-like and New Delhi metallo-beta-lactamase-1 (NDM-1). Microbiological cure was proven, and the patient died because of an ischaemic colitis secondary to Clostridium difficile infection. Besides, several in vitro studies88–91 support the good activity of cefiderocol against OXA-48 producing Enterobacterales, with MIC50 and MIC90 ranging 0.25–0.5 mg/L, and 0.5–4 mg/L, respectively.
Table 5.
Summary of the Studies Investigating the Treatment of OXA-48 Producing Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Ceftazidime-Avibactam and Cefiderocol
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | CPE-Producing Pathogens and Molecular Profile | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime-avibactam | |||||||||
| Sousa et al, 201886 | Prospective observational, non-comparative | 57 | CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
16 cIAI 15 HAP/VAP 14 cUTI 6 CR-BSI 3 SSTI 1 meningitis 1 osteomyelitis 1 mediastinitis |
54 K. pneumoniae 2 E. coli 1 E. cloacae All OXA-48 |
ICU admission 38% Septic shock 35% Mechanical ventilation 30% Median APACHE II score 24 |
Overall clinical cure rate: 77% Mortality rate at 30-day: 22% |
10% relapse | CAZ-AVI showed similar clinical cure and survival rate in OXA-48 infection compared to KPC |
| Temkin et al, 201771 | Retrospective observational multicentric, non-comparative | 38 | CAZ-AVI 100% target therapy |
15 cIAI 7 HAP 4 SSTI 3 cUTI 7 primary BSI or CR-BSI 2 endocarditis 3 osteomyelitis 2 surgical site infection 3 Others |
34 K. pneumoniae 1 K. oxytoca 1 E. coli 2 P. aeruginosa 23 KPC 13 OXA-48 |
Septic shock 44.7% Mechanical ventilation 36.8% Immunosuppression 23% |
Overall mortality rate: 39.5% Overall clinical/microbiological cure rate: 73.7% |
5.3% relapse | A trend to lower documented microbiological cure (25% vs 75%; p=0.07) and survival to hospital discharge (22.7% vs 77.3%; p=0.07) was found for the treatment of OXA-48 compared to KPC infections with CAZ-AVI |
| Alraddadi et al, 201984 | Retrospective observational cohort, comparative | 38 (10 CAZ-AVI vs 28 other agents) |
CAZ-AVI 2 g /0.5 g q8h 100% target therapy |
5 HAP 3 cUTI 3 cIAI 2 SSTI 1 CR-BSI |
7 K. pneumoniae 3 E. coli 8 OXA-48 1 NDM 1 NA |
Transplant recipient 50% | Overall clinical cure rate: 80% vs 53.6% Overall mortality rate: 50% vs 57.1% |
20% relapse | No difference in clinical cure (75% vs 40%; p=0.21) and mortality rate (37.5% vs 50%; p=0.69) after restricting analysis on patients with OXA-48 infections |
| De la Calle et al, 201985 | Retrospective observational, non-comparative | 24 | CAZ-AVI 2 g /0.5 g q8h 100% target therapy Dose adjustment for renal impairment according to manufacturer’s instruction |
7 cIAI 6 cUTI 5 HAP 4 SSTI 1 meningitis 1 CR-BSI |
23 K. pneumoniae 1 E. coli All OXA-48 (range MIC CAZ-AVI 0.5–1 mg/L) |
ICU admission 33.3% Septic shock 16.7% Mean SOFA score 3.3 Immunosuppression 21.7% Solid organ transplantation 21.7% |
Overall clinical cure rate: 62.5% Mortality rate at 30-day: 8.3% |
29.2% relapse | CAZ-AVI showed similar clinical cure and survival rate in OXA-48 infection compared to KPC |
| Cefiderocol | |||||||||
| Contreras et al, 201987 | Case report | 1 | Cefiderocol 1.5 g q12h |
bacteraemic cIAI |
K. pneumoniae NDM-1-OXA-48 |
ICU admission CRRT/ECMO Kidney transplant recipient |
Clinical and microbiological cure, but death due to ischaemic colitis | No relapse | |
| Dobias et al, 201788 | In vitro study | 154 OXA-48 isolates | 88 K. pneumoniae, 42 E. coli, 24 Enterobacter spp. Cefiderocol MIC range: 0.03–64 (MIC50 0.25 mg/L; MIC90: 2 mg/L) | ||||||
| Delgado-Valverde et al, 202091 | In vitro study | 57 OXA-48 isolates | 25 ST11/OXA-48 + CTX-M-15 K. pneumoniae. Cefiderocol MIC range ≤0.03–4 (MIC50 0.25 mg/L; MIC90: 2 mg/L) 25 ST15/OXA-48 + CTX-M-15 K. pneumoniae. Cefiderocol MIC range ≤0.03–4 (MIC50 0.25 mg/L; MIC90: 4 mg/L) 3 ST147/OXA-48 K. pneumoniae. Cefiderocol MIC range 0.06–0.5 (MIC50 0.25 mg/L; MIC90: 0.5 mg/L) 4 ST392/OXA-48 + CTX-M-15 K. pneumoniae. Cefiderocol MIC range 0.06–1 (MIC50 0.25 mg/L; MIC90: 1 mg/L) |
||||||
| Kazmierczak et al, 201989 | In vitro study | 32 OXA-48 isolates | 21 K. pneumoniae, 4 E. cloacae, 3 E. coli, 3 C. freundii, 1 K. Oxytoca. Cefiderocol MIC range: 0.03–4 (MIC50 0.5 mg/L; MIC90: 4 mg/L) | ||||||
| Jacobs et al, 201990 | In vitro study | 7 OXA-48 isolates | 7 K. pneumoniae. Cefiderocol MIC range ≤0.03–1 (MIC50 0.25 mg/L; MIC90: 1 mg/L) | ||||||
Abbreviations: BSIs, bloodstream infections; CAZ-AVI, ceftazidime-avibactam; cIAI, complicated intra abdominal infections; CR-BSI, catheter-related bloodstream infections; CRRT, continuous renal replacement therapy; cUTI, complicated urinary tract infection; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MIC, minimum inhibitory concentration; NA, not available; NDM, New-Delhi metallo-beta-lactamase; SSTI, skin and soft tissue infections; VAP, ventilator-associated pneumonia.
Metallo-Beta-Lactamase (MBL)-Positive Enterobacterales
Recommendations are depicted in Figure 2, panel A.6. Ceftazidime-avibactam (2.5g LD followed by 2.5g q8h over 8h CI) plus aztreonam (2g q8h over 8h CI after 2g LD) is the recommended first-line therapy for the management of BSIs, cIAIs, and cUTIs caused by New-Delhi metallo beta-lactamases (NDM)-producing Enterobacterales. Cefiderocol (2g LD followed by 2g q8h over 8h CI) is recommended for the treatment of infections caused by Verona Integron-encoded metallo-beta-lactamase (VIM)-producing and/or imipenemase (IMP)-producing Enterobacterales. Fosfomycin (6g LD followed by 16g CI) plus high-dose meropenem (1.5–2g q6h over 6h CI after 2g LD) could be an alternative option for both NDM-producing and VIM-IMP-producing Enterobacterales. MBLs are associated with extremely-drug-resistant (XDR) phenotypes, as they may hydrolyse the vast majority of currently available beta-lactams.92 A summary of the studies evaluating the efficacy of these antibiotics in the setting is provided in Table 6. One prospective observational study, two case series and five case reports suggest the efficacy of the combination therapy ceftazidime-avibactam plus aztreonam in the treatment of critically ill patients affected by NDM infections (mainly expressed by Klebsiella pneumoniae).93–100 Falcone et al93 compared in a prospective observational study 52 patients receiving the combination ceftazidime-avibactam plus aztreonam with 50 subjects receiving other active antibiotics in the management of BSIs due to NDM-producing and/or VIM-producing Enterobacterales. Patients treated with ceftazidime-avibactam plus aztreonam showed significantly lower mortality rate (19.2% vs 44%; p=0.007) and clinical failure rate (25% vs 52%; p=0.005). Shaw et al94 reported a case series of ceftazidime-avibactam plus aztreonam for the treatment of an outbreak caused by NDM-1/OXA-48-producing Klebsiella pneumoniae strain. Among 10 treated patients, 4 were solid organ transplant recipients, and half had bacteraemic infections (including cUTIs and cIAIs). Overall clinical cure rate at 30-day was 60%, and three patients died. Cairns et al101 reported a case series of four immunocompromised patients affected by IMP-4-producing Enterobacter cloacae infections successfully treated with ceftazidime-avibactam plus aztreonam, and relapse occurred only in one case. In regard to cefiderocol, only one case reported its role in the management of a secondary BSI caused by carbapenem-resistant K. pneumoniae co-producing NDM-1 and OXA-48-like beta-lactamases.87 However, several in vitro studies support the good activity of cefiderocol against NDM-producing Enterobacterales (MIC50: 1–4 mg/L and MIC90: 4–8 mg/L, susceptibility rate of 41–72.1%), and IMP-VIM-positive isolates (MIC50 1 mg/L, MIC90 4 mg/L, susceptibility rate of 80.9–95.7%).88,89,102 In regard to the combination of fosfomycin with high-dose meropenem, only one case documented the efficacy of this combo in a kidney transplant recipient affected by bacteraemic cUTI due to NDM-1-producing Morganella morganii.103 An in vitro study showed the synergistic effect of this combination against 10 NDM-producing Klebsiella pneumoniae strains (including five isolates co-producing OXA-48 carbapenemases).104
Table 6.
Summary of the Studies Investigating the Treatment of Metallo-Beta-Lactamase (MBL) Producing Enterobacterales Bloodstream Infections (BSIs), Complicated Intraabdominal (cIAIs) and Urinary Tract Infections (cUTIs) with Aztreonam-Avibactam, Cefiderocol and Combination Therapy with Meropenem and Fosfomycin
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | CPE-Producing Pathogens and Molecular Profile | Severity | Clinical Outcomes | Relapse Rate – Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| NDM+ pathogens | |||||||||
| Ceftazidime-avibactam + Aztreonam | |||||||||
| Falcone et al, 202093 | Prospective observational, multicenter with propensity score analysis | 102 (52 CAZ-AVI + Aztreonam vs 50 other active antibiotics) |
CAZ-AVI (2.5 g q8h – 50% of cases CI) + Aztreonam (2 g q8h) vs Colistin (LD 9 MU – MD 4.5 MU q12h); Tigecycline (LD 100 mg – MD 50 mg q12h); Fosfomycin (4–6 g q6h); Meropenem (2 g q8h); Gentamicin (3–5 mg/kg/day) |
All BSIs 33 cUTI 32 CR-BSI 12 SSTI 9 HAP/VAP 7 cIAI 14 unknown |
93 K. pneumoniae 5 Enterobacter spp 3 E. coli 1 M. morganii 82 NDM-producing 20 VIM-producing |
ICU admission 34.3% Solid organ transplantation 7.8% Septic shock 27.5% Mechanical ventilation 30.4% Median SOFA 4 |
30-day mortality rate: 19.2% vs 44% (p=0.007) |
NA | CAZ-AVI + ATM was associated with lower 30-day mortality (HR 0.37; P=0.01), lower clinical failure at day 14 (HR 0.30; P=0.002), and shorter length of stay (HR 0.49; P=0.007) |
| Shaw et al, 201794 | Case series | 10 | CAZ-AVI (0.94 g/day – 2.5 g q8h EI) + Aztreonam (1 g q8h – 2g q8h CI) |
4 cUTI 2 cIAI 2 HAP 1 CR-BSI 1 mediastinitis 50% bacteraemic |
10 K. pneumoniae 10 NDM-1/OXA-48/CTX-M-15 (outbreak) |
Mean SOFA: 3.7 Solid organ transplant recipient: 40% Immunocompromised: 50% |
Clinical cure rate at 30-day: 60% Mortality rate at 30-day: 30% |
Relapse 30% | |
| Benchetrit et al, 201995 | Case series | 2 | CAZ-AVI (0.94 g/day – 2.5 g/day EI) + Aztreonam 2 g q12-24 EI |
1 BSI 1 VAP |
2 K. pneumoniae 2 NDM-1 (MIC 0.032–0.064 mg/L) |
ICU admission: 100% Solid transplant recipient: 100% Immunocompromised: 100% |
Overall clinical cure rate: 100% Overall survival rate: 0% |
Relapse 100% | |
| Shah et al, 201996 | Case report | 1 | CAZ-AVI 0.94 g q12h + Aztreonam 1 g q8h |
bacteraemic cUTI |
K. pneumoniae No genotypification |
- | Clinical failure | - | |
| Hobson et al, 201997 | Case report | 1 | CAZ-AVI 150 mg/kg/day + Aztreonam (100 mg/kg/day) |
BSI |
Morganella morganii NDM-1 (MIC 0.016 mg/L) |
Haematological malignancy | Clinical and microbiological cure | No relapse at 6-month | |
| Sieswerda et al, 201998 | Case report | 1 | CAZ-AVI 2.5 g q8h CI + Aztreonam 1 g q8h EI |
cUTI |
K. pneumoniae NDM-1 (MIC 0.5 mg/L) |
Kidney transplant recipient | Clinical cure | Relapse at one month with the same strain. No difference in susceptibility |
|
| Yasmin et al, 202099 | Case report | 1 | CAZ-AVI 50 mg/kg q8h (EI 3h) + Aztreonam 50 mg/kg q8h |
BSI |
E. hormaechei NDM-1/KPC-4 (MIC 2/4 + 2 mg/L) |
Haematological malignancy | Clinical and microbiological cure | No relapse | |
| Bocanegra-Ibarias et al, 2020100 | Case report | 1 | CAZ-AVI 2.5 g q8h + Aztreonam 2 g q8h |
BSI |
K. pneumoniae NDM-1 (MIC 4/4 mg/L) |
Haematological malignancy | Clinical and microbiological cure | No relapse | |
| Cefiderocol | |||||||||
| Contreras et al, 201987 | Case report | 1 | Cefiderocol 1.5 g q12h |
bacteraemic cIAI |
K. pneumoniae NDM-1-OXA-48 |
ICU admission CRRT/ECMO Kidney transplant recipient |
Clinical and microbiological cure, but death due to ischaemic colitis | No relapse | |
| Dobias et al, 201788 | In vitro study | 134 NDM-VIM-IMP+ isolates | 67 E. coli, 38 K. pneumoniae, 29 Enterobacter spp. Cefiderocol MIC range: 0.03–64 (MIC50 1 mg/L; MIC90: 4 mg/L) | ||||||
| Mushtaq et al, 2020102 | In vitro study | 61 NDM isolates | 21 E. coli, 20 Klebsiella spp, 10 Enterobacter spp, 3 Citrobacter spp, 3 Providencia spp, 2 Morganella spp, 1 Serratia spp, 1 Proteus spp. Cefiderocol MIC range: 0.25–32 (MIC 2 S 41%; MIC 4 S 72.1%) | ||||||
| Kazmierczak et al, 201989 | In vitro study | 12 NDM isolates | 11 K. pneumoniae, 1 E. cloacae. Cefiderocol MIC range: 1–8 (MIC50 4 mg/L; MIC90: 8 mg/L) | ||||||
| Meropenem + fosfomycin | |||||||||
| Seija et al, 2015103 | Case report | 1 | Meropenem 2 g q8h EI + Fosfomycin 4 g q8h |
bacteraemic cUTI |
Morganella morganii NDM-1 |
Kidney transplant recipient | Clinical and microbiological cure | No relapse | |
| Sengel et al, 2020104 | In vitro study | 10 NDM isolates (5 NDM + 5 OXA-48/NDM) |
10 K. pneumoniae. Synergic activity in 100% of isolates | ||||||
| IMP/VIM+ pathogens | |||||||||
| Ceftazidime-avibactam + Aztreonam | |||||||||
| Cairns et al, 2020101 | Case series | 4 | CAZ-AVI 0.94 q12h - 2.5 g q8h + Aztreonam 1.5–2 g q8h |
2 bacteraemic cUTI 1 sternal osteomyelitis 1 CR-BSI |
4 E. cloacae IMP-4 (MIC 0.125–0.25 mg/L) |
ICU admission 50% 3 Solid organ transplant recipients 1 Haematological malignancy |
Clinical and microbiological cure: 100% |
Relapse 25% | |
| Cefiderocol | |||||||||
| Mushtaq et al, 2020102 | In vitro study | 62 VIM/IMP+ isolates | 22 Klebsiella spp, 20 E. coli, 12 Enterobacter spp, 8 Citrobacter spp. Cefiderocol MIC range: 0.03–8 (MIC 2 S 80.9%; MIC 4 S 95.7%) | ||||||
| Kazmierczak et al, 201989 | In vitro study | 27 VIM+ isolates | 8 K. pneumoniae, 7 E. cloacae, 7 C. freundii, 3 S. marcescens, 1 K. Oxytoca, 1 C. amanolaticus. Cefiderocol MIC range: 0.12–4 (MIC50 1 mg/L; MIC90: 4 mg/L) | ||||||
Abbreviations: BSIs, bloodstream infections; CAZ-AVI, ceftazidime-avibactam; CI, continuous infusion; cIAI, complicated intra abdominal infections; CR-BSI, catheter-related bloodstream infections; CRRT, continuous renal replacement therapy; cUTI, complicated urinary tract infection; ECMO, extracorporeal membrane oxygenation; EI, extended infusion; HR, hazard ratio; ICU, intensive care unit; LD, loading dose; MD, maintenance dose; MIC, minimum inhibitory concentration; NA, not available; NDM, New-Delhi metallo-beta-lactamase; SSTI, skin and soft tissue infections; VAP, ventilator-associated pneumonia; VIM, Verona-integrase metallo-beta-lactamase
Overview of Recommendations
The widespread diffusion of CRE isolates and the different genotypes of resistance of Enterobacterales requires careful attention for the right place in therapy of novel beta-lactams and the prompt adoption of strategies for sparing the broadest-spectrum antibiotics whenever possible. Ampicillin-sulbactam and ceftriaxone still represent the first-choice treatment of BSIs, cIAIs, or cUTIs caused by multi-susceptible Enterobacterales. Piperacillin-tazobactam should be recommended for the treatment of BSIs, cIAIs, or cUTIs caused by ESBL-producing Enterobacterales with an MIC ≤8 mg/L, especially in low-risk patients.105 In this scenario, altered dosing strategies based on high-doses administered by CI may maximize clinical efficacy of piperacillin-tazobactam against ESBL isolates, and this approach has been recently suggested by the EUCAST as well.105 Ertapenem was not recommended for the treatment of ESBL-producing infections in critically ill patients, as some studies106,107 showed that among patients affected by septic shock treatment with ertapenem was associated with higher mortality rate compared to other carbapenems. Cefepime may represent an effective “carbapenem-sparing” strategy for the treatment of infections caused by AmpC-producing Enterobacterales with an MIC ≤1 mg/L.108 Hopefully, some ongoing studies (namely MERINO-2, MERINO-3, PETERPEN, and FOREST studies) could better clarify the role of old and novel BL/BLIs in the management of critically ill patients affected by ESBL-producing Enterobacterales infections. Ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam, and cefiderocol may represent first-line choices for the management of critically ill patients affected by CRE infections expressing class A (eg, KPC) or D (eg, OXA-48) beta-lactamases.76 These agents should be administered in prolonged or continuous infusion as well to maximize efficacy and clinical outcome.75,109 Monotherapy with novel BL and BL/BLIs should always be pursued in these settings considering that comparative studies showed no significant advantage in efficacy of combination therapy vs monotherapy.110,111 Against MBL-producers, the use of ceftazidime-avibactam plus aztreonam may represent an effective strategy for the management of NDM-producing Enterobacterales infections, whereas cefiderocol should be reserved mainly to VIM- or IMP-producing Enterobacterales infections.76 This latter recommendations is justified by the fact that the overall susceptibility rate of cefiderocol against NDM-producing Enterobacterales is <70%, and MIC50/MIC90 are 2–4-fold higher compared to VIM- or IMP-producing strains.89,102,112,113 Fosfomycin combined with high-dose meropenem could represent a valuable alternative against both types of MBL-producing Enterobacterales infections.
Overall, altered dosing strategies of beta-lactams based on CI administration are strongly recommended for attaining very aggressive PK/PD target of 100% fT>4-8xMIC.51,105 This approach may both maximize clinical efficacy and prevent the development of resistance.105 CI is feasible with a unique daily solution infused over 24 hours for those drugs that are stable in aqueous solution at room temperature for ≥ 1 day (eg, piperacillin-tazobactam, ceftolozane-tazobactam). Otherwise, for those drugs that are stable in aqueous solution at room temperature for 6–12h, CI may be granted through reconstitution of the aqueous solution every 6–8h and infusion over 6–8h (eg, meropenem, ampicillin/sulbactam, ceftazidime/avibactam).
Clinicians must be aware that the antibiotic dosing regimens that we recommended throughout the manuscript are focused only on treatment of patients with normal renal function. It should not be overlooked that the pharmacokinetics of hydrophilic antimicrobial agents, namely beta-lactams and fosfomycin, may be affected among critically ill patients by several pathophysiological conditions that may alter volume of distribution and/or renal clearance. Consequently, dose adjustments are needed in critically ill renal patients, especially among those with transient acute kidney injury, augmented renal clearance, and/or undergoing renal replacement therapy.114 Finally, it should be mentioned that for the treatment of patients with well-documented life-threatening beta-lactam allergies, alternative agents s should be considered. Fluoroquinolones, aminoglycosides and colistin could be helpful in these cases depending on the susceptibility pattern of the isolated Enterobacterales.
Conclusions
In an era characterized by the widespread diffusion of MDR Gram-negative pathogens and by the incremental spread of antibiotic resistance, implementation of a multidisciplinary approach focused at targeted therapy in critically ill patients has become a real necessity. This could simultaneously allow to promptly revise inappropriate/unnecessary antibiotic regimens, to implement “carbapenem-sparing” strategies based on monotherapy with traditional and/or novel beta-lactams and, whenever applicable, to optimize antibiotic exposure in each single patient by means of real-time TDM guided approach. It is expected that these strategies could be helpful either in improving clinical outcome or in containing the spread of antimicrobial resistance in the ICU setting. It should be noted that the availability of rapid diagnostic technologies, based on molecular methods, that can reveal the presence of clinically-relevant resistance determinants such as the main ESBL and carbapenemase genes can be very useful to shorten the time for revision of empiric therapy according to the proposed algorithms.
Disclosure
B. Viaggi participated in advisory boards and in speaker’s bureau for, and received research contracts, contributions and study events from Abbott, Accelerate Diagnostics, Ada, Alifax, Angelini, Becton Dickinson, Bellco, Biomerieux, Biotest, Cepheid, Correvio, Gilead, Menarini, MSD Italia, Nordic Pharma, Pfizer, Shionogi, Thermo Fisher Scientific; G.M. Rossolini participated in advisory boards and speaker’s bureau for, and received research contracts, contributions and travel grants from Accelerate, Angelini, Arrow, Beckman Biomedical Service, Coulter, Becton-Dickinson, bioMérieux, Cepheid, Hain Life Sciences, Menarini, Meridian, MSD, Nordic Pharma, Pfizer, Qiagen, Q-linea, Qpex, Quidel, Qvella, Roche, Seegene, Set-Lance, Shionogi, Symcel, ThermoFisher, VenatorX, Zambon; F. Pea participated in speaker bureau for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis, and in advisory board for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfizer, and Thermo-Fisher. P. Viale has served as a consultant for Biomerieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureau for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma and Pfizer. The authors report no other conflicts of interest in this work.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623 [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
- 3.Vincent J-L, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi: 10.1001/jama.2020.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28(2):108–121. doi: 10.1007/s00134-001-1143-z [DOI] [PubMed] [Google Scholar]
- 5.MacVane SH. Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med. 2017;32(1):25–37. doi: 10.1177/0885066615619895 [DOI] [PubMed] [Google Scholar]
- 6.Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis. 2014;78(4):443–448. doi: 10.1016/j.diagmicrobio.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 7.Timsit J-F, Bassetti M, Cremer O, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45(2):172–189. doi: 10.1007/s00134-019-05520-5 [DOI] [PubMed] [Google Scholar]
- 8.Detsis M, Karanika S, Mylonakis E. ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):705–714. doi: 10.1097/CCM.0000000000002253 [DOI] [PubMed] [Google Scholar]
- 9.Thabit AK, Crandon JL, Nicolau DP. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother. 2015;16(2):159–177. doi: 10.1517/14656566.2015.993381 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248. doi: 10.1378/chest.09-0087 [DOI] [PubMed] [Google Scholar]
- 11.Ulldemolins M, Nuvials X, Palomar M, Masclans JR, Rello J. Appropriateness is critical. Crit Care Clin. 2011;27(1):35–51. doi: 10.1016/j.ccc.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 13.Pea F, Viale P. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock--does the dose matter? Crit Care. 2009;13(3):214. doi: 10.1186/cc7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viale P, Tedeschi S, Scudeller L, et al. Infectious Diseases Team for the Early Management of Severe Sepsis and Septic Shock in the Emergency Department. Clin Infect Dis. 2017;65(8):1253–1259. doi: 10.1093/cid/cix548 [DOI] [PubMed] [Google Scholar]
- 15.Gatti M, Gasparini LE, Laratta M, et al. Intensive multidisciplinary management in critical care patients affected by severe necrotizing soft tissue infections: a cooperative method to improve the efficacy of treatment. Eur J Clin Microbiol Infect Dis. 2019;38(6):1153–1162. doi: 10.1007/s10096-019-03521-2 [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Zhang M, Huang P, et al. Novel β-lactam/β-lactamase inhibitors versus alternative antibiotics for the treatment of complicated intra-abdominal infection and complicated urinary tract infection: a meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther. 2018;16(2):111–120. doi: 10.1080/14787210.2018.1429912 [DOI] [PubMed] [Google Scholar]
- 17.Gudiol C, Cuervo G, Carratalà J. Optimizing therapy of bloodstream infection due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Curr Opin Crit Care. 2019;25(5):438–448. doi: 10.1097/MCC.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Valverde M, Torres E, Valiente-Mendez A, et al. Impact of the MIC of piperacillin/tazobactam on the outcome for patients with bacteraemia due to Enterobacteriaceae: the Bacteraemia-MIC project. J Antimicrob Chemother. 2016;71(2):521–530. doi: 10.1093/jac/dkv362 [DOI] [PubMed] [Google Scholar]
- 19.Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21(4):125–127. doi: 10.1136/ebmed-2016-110401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckmann C, Dryden M, Montravers P, Kozlov R, Sganga G. Antimicrobial treatment of “complicated” intra-abdominal infections and the new IDSA guidelines? a commentary and an alternative European approach according to clinical definitions. Eur J Med Res. 2011;16(3):115–126. doi: 10.1186/2047-783x-16-3-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi: 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 22.Walker AP, Nichols RL, Wilson RF, et al. Efficacy of a beta-lactamase inhibitor combination for serious intraabdominal infections. Ann Surg. 1993;217(2):115–121. doi: 10.1097/00000658-199302000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnon PS, Neuhauser MM. Efficacy and cost of ampicillin-sulbactam and ticarcillin-clavulanate in the treatment of hospitalized patients with bacterial infections. Pharmacotherapy. 1999;19(6):724–733. doi: 10.1592/phco.19.9.724.31537 [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein E, Lode H, Grassi C. Ceftazidime monotherapy vs. ceftriaxone/tobramycin for serious hospital-acquired gram-negative infections. Antibiotic Study Group. Clin Infect Dis. 1995;20(5):1217–1228. doi: 10.1093/clinids/20.5.1217 [DOI] [PubMed] [Google Scholar]
- 25.Tomera KM, Burdmann EA, Reyna OGP, et al. Ertapenem versus ceftriaxone followed by appropriate oral therapy for treatment of complicated urinary tract infections in adults: results of a prospective, randomized, double-blind multicenter study. Antimicrob Agents Chemother. 2002;46(9):2895–2900. doi: 10.1128/aac.46.9.2895-2900.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez-Cruz F, Jasovich A, Cajigas J, et al. A prospective, multicenter, randomized, double-blind study comparing ertapenem and ceftriaxone followed by appropriate oral therapy for complicated urinary tract infections in adults. Urology. 2002;60(1):16–22. doi: 10.1016/s0090-4295(02)01664-3 [DOI] [PubMed] [Google Scholar]
- 27.Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004;53(Suppl 2):ii67–74. doi: 10.1093/jac/dkh208 [DOI] [PubMed] [Google Scholar]
- 28.Park DW, Peck KR, Chung MH, et al. Comparison of ertapenem and ceftriaxone therapy for acute pyelonephritis and other complicated urinary tract infections in Korean adults: a randomized, double-blind, multicenter trial. J Korean Med Sci. 2012;27(5):476–483. doi: 10.3346/jkms.2012.27.5.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson DL, Henderson A, Harris PNA. Current evidence for therapy of ceftriaxone-resistant Gram-negative bacteremia. Curr Opin Infect Dis. 2020;33(1):78–85. doi: 10.1097/QCO.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 30.Papadimitriou-Olivgeris M, Bartzavali C, Lambropoulou A, et al. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J Antimicrob Chemother. 2019;74(7):2051–2054. doi: 10.1093/jac/dkz125 [DOI] [PubMed] [Google Scholar]
- 31.Harris PNA, Tambyah PA, Lye DC, et al. Effect of Piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320(10):984–994. doi: 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Kahlmeter G. Antibiotics for Ceftriaxone-resistant gram-negative bacterial bloodstream infections. JAMA. 2019;321(6):612–613. doi: 10.1001/jama.2018.19345 [DOI] [PubMed] [Google Scholar]
- 33.Livermore DM, Day M, Cleary P, et al. OXA-1 β-lactamase and non-susceptibility to penicillin/β-lactamase inhibitor combinations among ESBL-producing Escherichia coli. J Antimicrob Chemother. 2019;74(2):326–333. doi: 10.1093/jac/dky453 [DOI] [PubMed] [Google Scholar]
- 34.Sfeir MM, Askin G, Christos P. Beta-lactam/beta-lactamase inhibitors versus carbapenem for bloodstream infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52(5):554–570. doi: 10.1016/j.ijantimicag.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Á P. Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54(2):167–174. doi: 10.1093/cid/cir790 [DOI] [PubMed] [Google Scholar]
- 36.Kang C-I, Park SY, Chung DR, Peck KR, Song J-H. Piperacillin-tazobactam as an initial empirical therapy of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Infect. 2012;64(5):533–534. doi: 10.1016/j.jinf.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 37.Harris PNA, Yin M, Jureen R, et al. Comparable outcomes for β-lactam/β-lactamase inhibitor combinations and carbapenems in definitive treatment of bloodstream infections caused by cefotaxime-resistant Escherichia coli or Klebsiella pneumoniae. Antimicrob Resist Infect Control. 2015;4:14. doi: 10.1186/s13756-015-0055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, et al. A multinational, preregistered cohort study of β-Lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2016;60(7):4159–4169. doi: 10.1128/AAC.00365-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng TM, Khong WX, Harris PNA, et al. Empiric Piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing enterobacteriaceae. PLoS One. 2016;11(4):e0153696. doi: 10.1371/journal.pone.0153696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gudiol C, Royo-Cebrecos C, Abdala E, et al. Efficacy of β-Lactam/β-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum-β-lactamase-producing enterobacteriaceae in hematological patients with neutropenia. Antimicrob Agents Chemother. 2017;61:8. doi: 10.1128/AAC.00164-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon YK, Kim JH, Sohn JW, Yang KS, Kim MJ. Role of piperacillin/tazobactam as a carbapenem-sparing antibiotic for treatment of acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2017;49(4):410–415. doi: 10.1016/j.ijantimicag.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 42.Ko J-H, Lee NR, Joo E-J, et al. Appropriate non-carbapenems are not inferior to carbapenems as initial empirical therapy for bacteremia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: a propensity score weighted multicenter cohort study. Eur J Clin Microbiol Infect Dis. 2018;37(2):305–311. doi: 10.1007/s10096-017-3133-2 [DOI] [PubMed] [Google Scholar]
- 43.Benanti GE, Brown ART, Shigle TL, et al. Carbapenem versus cefepime or piperacillin-tazobactam for empiric treatment of bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli in patients with hematologic malignancy. Antimicrob Agents Chemother. 2019;63:2. doi: 10.1128/AAC.01813-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John R, Colley P, Nguyen HL, Berhe M. Outcomes analysis in patients with extended-spectrum beta-lactamase bacteremia empirically treated with piperacillin/tazobactam versus carbapenems. Proc (Bayl Univ Med Cent). 2019;32(2):187–191. doi: 10.1080/08998280.2019.1582466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasir N, Ahmed S, Razi S, Awan S, Mahmood SF. Risk factors for mortality of patients with ceftriaxone resistant E. coli bacteremia receiving carbapenem versus beta lactam/beta lactamase inhibitor therapy. BMC Res Notes. 2019;12(1):611. doi: 10.1186/s13104-019-4648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharara SL, Amoah J, Pana ZD, Simner PJ, Cosgrove SE, Tamma PD. Is Piperacillin-Tazobactam Effective for the Treatment of Pyelonephritis Caused by Extended-Spectrum β-Lactamase-Producing Organisms? Clin Infect Dis. 2020;71(8):e331–e337. doi: 10.1093/cid/ciz1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo YB, Lee J, Kim YK, et al. Randomized controlled trial of piperacillin-tazobactam, cefepime and ertapenem for the treatment of urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli. BMC Infect Dis. 2017;17(1):404. doi: 10.1186/s12879-017-2502-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319–1325. doi: 10.1093/cid/civ003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ofer-Friedman H, Shefler C, Sharma S, et al. Carbapenems versus piperacillin-tazobactam for bloodstream infections of nonurinary source caused by extended-spectrum beta-lactamase-producing enterobacteriaceae. Infect Control Hosp Epidemiol. 2015;36(8):981–985. doi: 10.1017/ice.2015.101 [DOI] [PubMed] [Google Scholar]
- 50.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108–120. doi: 10.1016/S1473-3099(17)30615-1 [DOI] [PubMed] [Google Scholar]
- 51.Thabit AK, Hobbs ALV, Guzman OE, Shea KM. the pharmacodynamics of prolonged infusion β-lactams for the treatment of pseudomonas aeruginosa infections: a systematic review. Clin Ther. 2019;41(11):2397–2415.e8. doi: 10.1016/j.clinthera.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 52.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, Phase 3 trial (ASPECT-cUTI). Lancet. 2015;385(9981):1949–1956. doi: 10.1016/S0140-6736(14)62220-0 [DOI] [PubMed] [Google Scholar]
- 53.Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/Tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015;60(10):1462–1471. doi: 10.1093/cid/civ097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam Versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a Phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–762. doi: 10.1093/cid/ciw378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–1389. doi: 10.1093/cid/ciw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin X, Tran BG, Kim MJ, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49(5):579–588. doi: 10.1016/j.ijantimicag.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 57.Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6):661–673. doi: 10.1016/S1473-3099(16)30004-4 [DOI] [PubMed] [Google Scholar]
- 58.Meini S, Tascini C, Cei M, Sozio E, Rossolini GM. AmpC β-lactamase-producing Enterobacterales: what a clinician should know. Infection. 2019;47(3):363–375. doi: 10.1007/s15010-019-01291-9 [DOI] [PubMed] [Google Scholar]
- 59.Harris PNA, Wei JY, Shen AW, et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother. 2016;71(2):296–306. doi: 10.1093/jac/dkv346 [DOI] [PubMed] [Google Scholar]
- 60.Blanchette LM, Kuti JL, Nicolau DP, Nailor MD. Clinical comparison of ertapenem and cefepime for treatment of infections caused by AmpC beta-lactamase-producing Enterobacteriaceae. Scand J Infect Dis. 2014;46(11):803–808. doi: 10.3109/00365548.2014.954262 [DOI] [PubMed] [Google Scholar]
- 61.Tamma PD, Girdwood SCT, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57(6):781–788. doi: 10.1093/cid/cit395 [DOI] [PubMed] [Google Scholar]
- 62.Siedner MJ, Galar A, Guzmán-Suarez BB, et al. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis. 2014;58(11):1554–1563. doi: 10.1093/cid/ciu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hilty M, Sendi P, Seiffert SN, et al. Characterisation and clinical features of Enterobacter cloacae bloodstream infections occurring at a tertiary care university hospital in Switzerland: is cefepime adequate therapy? Int J Antimicrob Agents. 2013;41(3):236–249. doi: 10.1016/j.ijantimicag.2012.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan SH, Ng TM, Chew KL, et al. Outcomes of treating AmpC-producing Enterobacterales bacteraemia with carbapenems vs. non-carbapenems. Int J Antimicrob Agents. 2020;55(2):105860. doi: 10.1016/j.ijantimicag.2019.105860 [DOI] [PubMed] [Google Scholar]
- 65.Lee N-Y, Lee -C-C, Li C-W, et al. Cefepime therapy for monomicrobial enterobacter cloacae bacteremia: unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother. 2015;59(12):7558–7563. doi: 10.1128/AAC.01477-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng L, Nelson BC, Mehta M, et al. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC β-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2017;61:6. doi: 10.1128/AAC.00276-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castón JJ, Lacort-Peralta I, Martín-Dávila P, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis. 2017;59:118–123. doi: 10.1016/j.ijid.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 69.Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68(3):355–364. doi: 10.1093/cid/ciy492 [DOI] [PubMed] [Google Scholar]
- 70.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-Avibactam is superior to other treatment regimens against carbapenem-resistant klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61:8. doi: 10.1128/AAC.00883-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temkin E, Torre-Cisneros J, Beovic B, et al. Ceftazidime-Avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother. 2017;61:2. doi: 10.1128/AAC.01964-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61:7. doi: 10.1128/AAC.00449-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62:5. doi: 10.1128/AAC.02497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tumbarello M, Raffaelli F, Giannella M, et al. Ceftazidime-avibactam use for KPC-Kp infections: a retrospective observational multicenter study. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab176 [DOI] [PubMed] [Google Scholar]
- 76.Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, New LL. β-Lactam-β-Lactamase Inhibitor Combinations. Clin Microbiol Rev. 2020;34:1. doi: 10.1128/CMR.00115-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7(4):439–455. doi: 10.1007/s40121-018-0214-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackley R, Roshdy D, Meredith J, et al. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother. 2020;64:5. doi: 10.1128/AAC.02313-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alosaimy S, Jorgensen SCJ, Lagnf AM, et al. Real-world multicenter analysis of clinical outcomes and safety of meropenem-vaborbactam in patients treated for serious gram-negative bacterial infections. Open Forum Infect Dis. 2020;7(3):ofaa051. doi: 10.1093/ofid/ofaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shields RK, McCreary EK, Marini RV, et al. Early experience with meropenem-vaborbactam for treatment of carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis. 2020;71(3):667–671. doi: 10.1093/cid/ciz1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70(9):1799–1808. doi: 10.1093/cid/ciz530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226–240. doi: 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 83.Stewart A, Harris P, Henderson A, Paterson D. Treatment of Infections by OXA-48-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62:11. doi: 10.1128/AAC.01195-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772. doi: 10.1186/s12879-019-4409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De la Calle C, Rodríguez O, Morata L, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2019;53(4):520–524. doi: 10.1016/j.ijantimicag.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 86.Sousa A, Pérez-Rodríguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3170–3175. doi: 10.1093/jac/dky295 [DOI] [PubMed] [Google Scholar]
- 87.Contreras DA, Fitzwater SP, Nanayakkara DD, et al. Coinfections of Two Strains of NDM-1- and OXA-232-Coproducing Klebsiella pneumoniae in a Kidney Transplant Patient. Antimicrob Agents Chemother. 2020;64:4. doi: 10.1128/AAC.00948-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis. 2017;36(12):2319–2327. doi: 10.1007/s10096-017-3063-z [DOI] [PubMed] [Google Scholar]
- 89.Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents. 2019;53(2):177–184. doi: 10.1016/j.ijantimicag.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 90.Jacobs MR, Abdelhamed AM, Good CE, et al. ARGONAUT-I: activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, Including Carbapenem-Resistant Nonfermenters and Enterobacteriaceae with Defined Extended-Spectrum β-Lactamases and Carbapenemases. Antimicrob Agents Chemother. 2019;63:1. doi: 10.1128/AAC.01801-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delgado-Valverde M, Conejo MDC, Serrano L, Fernández-Cuenca F, Pascual Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother. 2020;75(7):1840–1849. doi: 10.1093/jac/dkaa117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyd SE, Livermore DM, Hooper DC, Hope WW. Metallo-β-Lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother. 2020;64:10. doi: 10.1128/AAC.00397-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Falcone M, Daikos GL, Tiseo G, et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by MBL- producing Enterobacterales. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa586 [DOI] [PubMed] [Google Scholar]
- 94.Shaw E, Rombauts A, Tubau F, et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73(4):1104–1106. doi: 10.1093/jac/dkx496 [DOI] [PubMed] [Google Scholar]
- 95.Benchetrit L, Mathy V, Armand-Lefevre L, Bouadma L, Timsit J-F. Successful treatment of septic shock due to NDM-1-producing Klebsiella pneumoniae using ceftazidime/avibactam combined with aztreonam in solid organ transplant recipients: report of two cases. Int J Antimicrob Agents. 2020;55(1):105842. doi: 10.1016/j.ijantimicag.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 96.Shah PJ, Tran T, Emelogu F, Tariq F. Aztreonam, Ceftazidime/Avibactam, and Colistin Combination for the Management of Carbapenemase-Producing Klebsiella Pneumoniae Bacteremia: a Case Report. J Pharm Pract. 2019;897190019882262. doi: 10.1177/0897190019882262 [DOI] [PubMed] [Google Scholar]
- 97.Hobson CA, Bonacorsi S, Fahd M, et al. Successful Treatment of Bacteremia Due to NDM-1-Producing Morganella morganii with Aztreonam and Ceftazidime-Avibactam Combination in a Pediatric Patient with Hematologic Malignancy. Antimicrob Agents Chemother. 2019;63:2. doi: 10.1128/AAC.02463-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sieswerda E, van den Brand M, van den Berg RB, et al. Successful rescue treatment of sepsis due to a pandrug-resistant, NDM-producing Klebsiella pneumoniae using aztreonam powder for nebulizer solution as intravenous therapy in combination with ceftazidime/avibactam. J Antimicrob Chemother. 2020;75(3):773–775. doi: 10.1093/jac/dkz495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yasmin M, Fouts DE, Jacobs MR, et al. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase-4 and New Delhi Metallo-β-Lactamase-1. Clin Infect Dis. 2020;71(4):1095–1098. doi: 10.1093/cid/ciz1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bocanegra-Ibarias P, Camacho-Ortiz A, Garza-González E, Flores-Treviño S, Kim H, Perez-Alba E. Aztreonam plus ceftazidime-avibactam as treatment of NDM-1-producing Klebsiella pneumoniae bacteraemia in a neutropenic patient: last resort therapy? J Glob Antimicrob Resist. 2020;23:417–419. doi: 10.1016/j.jgar.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 101.Cairns KA, Hall V, Martin GE, et al. Treatment of invasive IMP-4 Enterobacter cloacae infection in transplant recipients using ceftazidime/avibactam with aztreonam: a case series and literature review. Transpl Infect Dis. 2020. doi: 10.1111/tid.13510 [DOI] [PubMed] [Google Scholar]
- 102.Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother. 2020;64:12. doi: 10.1128/AAC.01582-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seija V, Medina Presentado JC, Bado I, et al. Sepsis caused by New Delhi metallo-β-lactamase (blaNDM-1) and qnrD-producing Morganella morganii, treated successfully with fosfomycin and meropenem: case report and literature review. Int J Infect Dis. 2015;30:20–26. doi: 10.1016/j.ijid.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 104.Erturk Sengel B, Altinkanat Gelmez G, Soyletir G, Korten V. In vitro synergistic activity of fosfomycin in combination with meropenem, amikacin and colistin against OXA-48 and/or NDM-producing Klebsiella pneumoniae. J Chemother. 2020;32(5):237–243. doi: 10.1080/1120009X.2020.1745501 [DOI] [PubMed] [Google Scholar]
- 105.Tamma PD, The R-BJ. Use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin Infect Dis. 2017;64(7):972–980. doi: 10.1093/cid/cix034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins VL, Marchaim D, Pogue JM, et al. Efficacy of ertapenem for treatment of bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2012;56(4):2173–2177. doi: 10.1128/AAC.05913-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gutiérrez-Gutiérrez B, Bonomo RA, Carmeli Y, et al. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: a multinational pre-registered cohort study. J Antimicrob Chemother. 2016;71(6):1672–1680. doi: 10.1093/jac/dkv502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karaiskos I, Carbapenem-Sparing GH. Strategies for ESBL producers: when and how. Antibiotics (Basel). 2020;9:2. doi: 10.3390/antibiotics9020061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pilmis B, Petitjean G, Lesprit P, et al. Continuous infusion of ceftolozane/tazobactam is associated with a higher probability of target attainment in patients infected with Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2019;38(8):1457–1461. doi: 10.1007/s10096-019-03573-4 [DOI] [PubMed] [Google Scholar]
- 110.Meini S, Viaggi B, Tascini C. Mono vs. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: insights from the literature. Infection. 2021. doi: 10.1007/s15010-021-01577-x [DOI] [PubMed] [Google Scholar]
- 111.Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54(6):735–740. doi: 10.1016/j.ijantimicag.2019.08.025 [DOI] [PubMed] [Google Scholar]
- 112.Giacobbe DR, Ciacco E, Girmenia C, et al. Evaluating Cefiderocol in the Treatment of Multidrug-Resistant Gram-Negative Bacilli: a Review of the Emerging Data. Infect Drug Resist. 2020;13:4697–4711. doi: 10.2147/IDR.S205309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kohira N, Hackel MA, Ishioka Y, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist. 2020;22:738–741. doi: 10.1016/j.jgar.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 114.Gatti M, Pea F. Pharmacokinetic/pharmacodynamic target attainment in critically ill renal patients on antimicrobial usage: focus on novel beta-lactams and beta lactams/beta-lactamase inhibitors. Expert Rev Clin Pharmacol. 2021. doi: 10.1080/17512433.2021.1901574 [DOI] [PubMed] [Google Scholar]