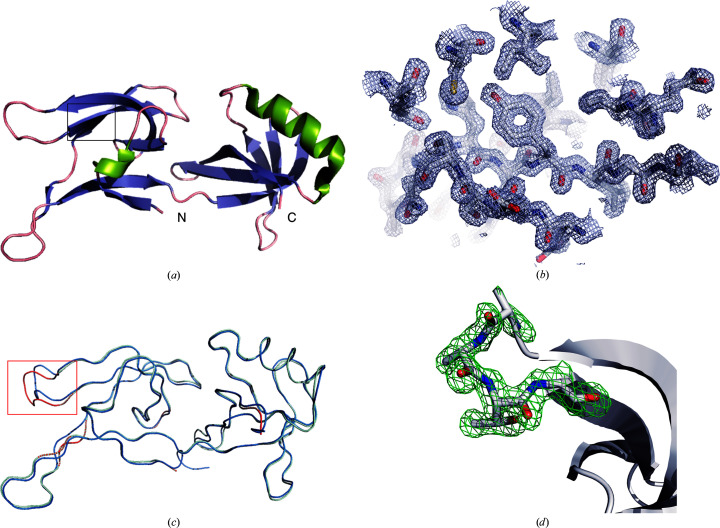
Figure 4.
Details of the HEX-1 structure. (a) Overall structure of HEX-1 in cartoon representation. (b) Representative region of the electron-density map of HEX-1 obtained by FT-SFX of intracellular crystals at 1.8 Å resolution from a single chip (PDB entry 7asx). The detailed region is marked by a black box in (a). (c) Structural homology of HEX-1 crystallized in insect cells and HEX-1 purified from E. coli and crystallized by sitting-drop vapour diffusion. A backbone representation of the HEX-1 structure (green) obtained from in cellulo diffraction is superimposed with that of the HEX-1 reference structure (PDB code 1khi; blue). The average r.m.s.d. is 0.47 Å for equivalent Cα atoms. The only region showing major structural differences with r.m.s.d.s above 0.6 Å is highlighted by the red box. (d) OMIT map of residues 62–65 of the FT-SFX HEX-1 structure with the same residues in stick representation. The F o − F c map (green) of the region of largest r.m.s.d.s with the reference structure, highlighted in (c), is contoured at 3σ.