Abstract
Primary gastrointestinal mucormycosis is a rare disease associated with an increased mortality and is rarely reported in an immunocompetent host. We report the first case of mucormycosis-associated colonic perforation in a COVID-19 patient with a favourable outcome. A 48-year-old healthy male doctor in home isolation due to COVID-19 was admitted to COVID-19 intensive care unit when his symptoms deteriorated. The patient was put on non-invasive ventilation (NIV) using Bilevel Positive Airway Pressure (BiPAP) and treatment given as per existing hospital protocol. The patient improved clinically, and was discharged on day 10 of admission. Two days later, he presented with acute gastrointestinal symptoms to the emergency department. A diagnosis of perforation peritonitis was made, the patient was stabilised and sigmoid colectomy with descending colon colostomy was done. A diagnosis of gastrointestinal mucormycosis was made and injectable antifungal was started. The patient was discharged after his general conditions improved.
Keywords: COVID-19, infections, infection (gastroenterology), adult intensive care, general surgery
Background
The COVID-19 may present with varied disease patterns, ranging from mild to moderate symptoms to acute respiratory distress syndrome.1 Hospital-acquired bacterial and fungal coinfections may develop during their stay in the hospital. Mucormycosis is a rare life-threatening fungal infection prevalent in those with diabetes mellitus, systemic corticosteroid therapy, neutropenia, haematological malignancy, stem cell transplant and immune-compromised condition.2 Depending on the anatomic location, it may present as distinctive syndromes, of which rhino-orbital-cerebral being the most common.3 4 Other less common sites are pulmonary, cutaneous, and rarely, gastrointestinal, renal and disseminated diseases. The common organs affected in the gastrointestinal tract include the stomach followed by the colon and ileum.5 Here, we are presenting a case of gastrointestinal mucormycosis in a patient following a recent COVID-19 infection. The patient had a good outcome because of an early clinical suspicion, timely diagnosis and prompt treatment.
Case presentation
A 48-year-old intensive care physician who was in-charge of a dedicated COVID-19 intensive therapy unit got infected with COVID-19 in the June of 2020. He had no previous known systemic illnesses. After being in home isolation for 5 days, his condition started deteriorating fast and was admitted to the COVID-19 intensive care unit (ICU). He complained of cough, high-grade fever of >103°F (39.4°C), tachycardia, tachypnoea, breathlessness, hypoxia and respiratory distress on admission. He did not have any comorbidities including diabetes, hypertension, obesity, cardiac or pulmonary disorder. His investigations were suggestive of severe COVID-19 infection with COVID-19 Reporting and Data System (CO-RAD) score of 22/25 on high-resolution computerised tomography (HRCT).6 He was treated with injectable piperacillin–tazobactam, doxycycline, low molecular heparin, remdesivir, tocilizumab, methylprednisolone and proton pump inhibitors as per institutional protocol. He also received vasopressin for haemodynamic support and later put on NIV support. His condition gradually stabilised and he was later discharged on day 17 of hospital admission.
Two days later, he presented to the emergency room (ER) with acute abdominal pain, vomiting, obstipation and massive lower gastrointestinal (GI) bleed. His abdominal pain was sudden, severe and progressively increasing. He also had multiple episodes of non-projectile vomiting for 1 day. He had one episode of the lower GI bleed with the passage of both fresh and altered blood on the morning of admission and then another episode of lower GI bleed in the ER.
His general condition was poor and he had tachycardia (130 beats per min), low volume pulse and hypotension (blood pressure: 90/48 mm Hg). He was afebrile and had not passed urine for more than 12 hours. He was tachypnoeic (respiratory rate, 28 per min), had oxygen saturation (SpO2) of 92% on pulse oximetry and bilateral basal crepitations on chest auscultation. His abdomen was distended, tense, with rigidity and rebound tenderness suggestive of peritonitis. He was resuscitated in ER and then shifted to ICU where his resuscitation was continued and norepinephrine (0.2–0.4 µg/kg/min) was started.
Investigations
All routine investigations done showed a haemoglobin of 7.8 gm/dL, total white cell count of 35 000/mm3 with 89% neutrophils and 15% leukocytes, platelet count of 1.2 lacs/mm3, serum urea 145 mg/dL, serum creatinine 1.8 mg/dL, elevated CRP (90 mg/L), aspartate aminotransferase (150) and aminotransferase (120). The rest of the laboratory parameters including electrolytes, HIV and hepatitis B virus negative and coagulation parameters were normal. His HRCT chest had bilateral pneumonitis (figure 1) with a 20/25 CT severity score and a CO-RAD score of 5 and the quantitative COVID-19 IgG antibody was 5 IU.
Figure 1.
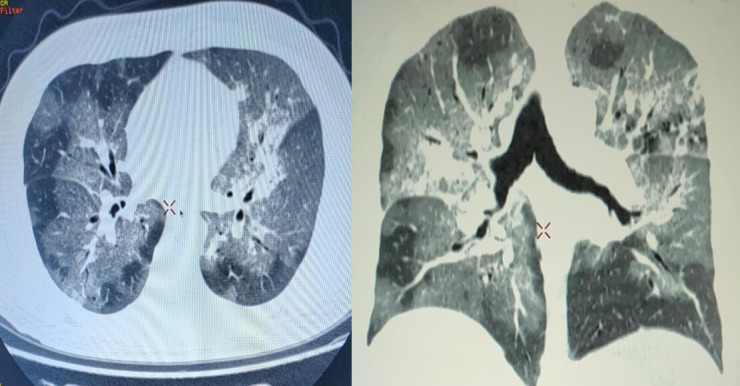
HRCT chest showing discrete and confluent areas of ground glass haze and interlobular thickening confirming consolidations predominantly in peripheral parenchyma of bilateral lungs. The findings were suggestive of viral infection with CT COVID severity score of 22/25. HRCT, high-resolution computerised tomography.
Differential diagnosis
A tentative diagnosis of faecal perforation peritonitis with haemorrhagic gangrene was made based on clinical presentation and radiological investigations CT scan which showed gas under the diaphragm (figure 2). The patient had sepsis, hypovolemic shock with acute kidney injury. The initial differential diagnosis included drug-related (tocilizumab related?) vasculitis, ischaemic colitis, cytomegalovirus invasive disease and tubercular or fungal aetiology leading to perforation of his sigmoid colon.
Figure 2.
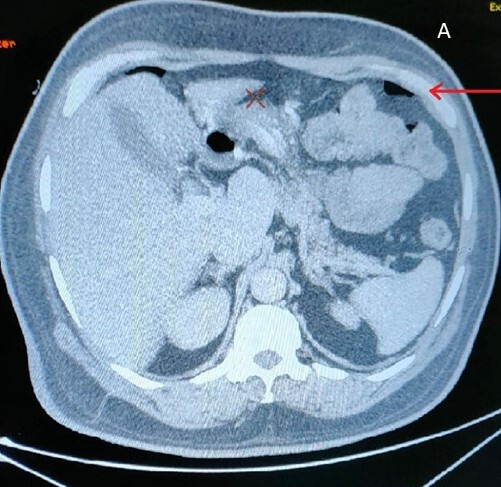
CT scan which showed gas under the diaphragm.
Treatment
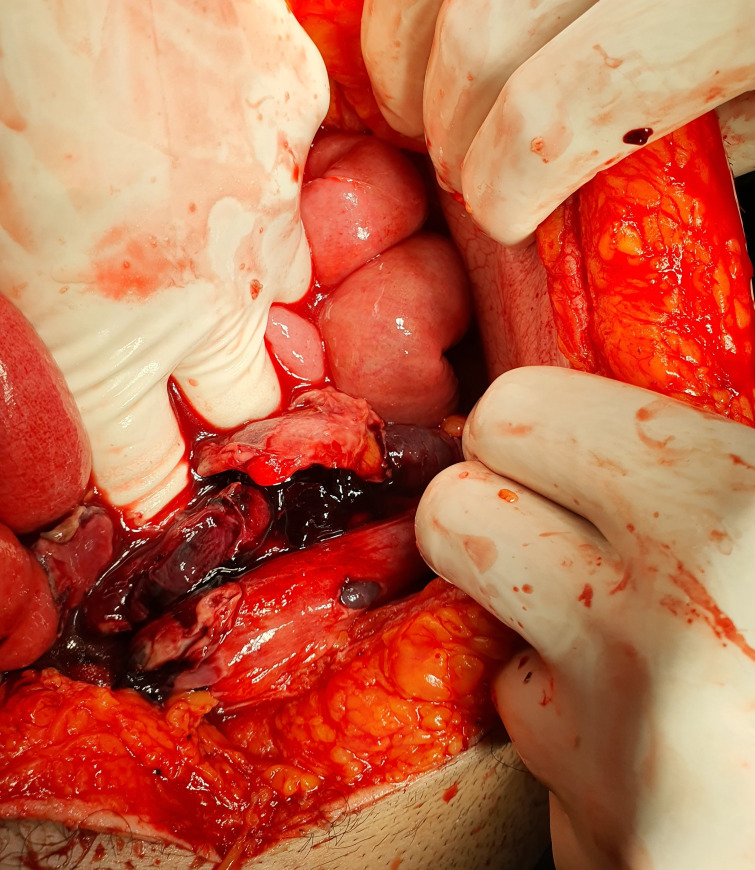
He was shifted to the operation room for an emergency life-saving laparotomy for perforation peritonitis. Surgical findings revealed faecal perforation peritonitis with 2 litres of feco-purulent collection in the abdominal cavity, multiple sigmoid colon perforations, haemorrhagic gangrene of sigmoid colon along with intraabdominal and intraluminal blood (figure 3).
Figure 3.
Sigmoid colon perforations with haemorrhagic gangrene.
He underwent sigmoid colectomy with descending colon colostomy and upper rectal mucus fistula. The specimen was sent for pathological examination. In the postoperative period, he continued to have tachycardia, tachypnoea, hypotension, low urine output and marginal saturation. His condition deteriorated and high dose inotropes were started and was put on NIV. Subsequently on day 4, an initial pre-emptive histopathology report showed an irregular wide, aseptate, broad, fungal hyphae with right-angle branching suggestive of mucor in sigmoid colectomy specimen. There were no caseation, vasculitis or viral inclusions. He was immediately started on liposomal amphotericin B, initially at 3 mg/kg which was progressively increased to 5 mg/kg in 2 days for a total of 7 days. Also, tablet posaconazole (400 mg, two times per day) was continued in the postoperative period for 14 days.
Outcome and follow-up
He showed progressive improvement with stabilisation of vitals, decreasing vasopressor requirement, improvement of general well-being and was later discharged on the postoperative day 11. He recovered well and was under strict follow-up. He was taken up for his stoma closure after 3 months of index surgery. During this time, the distal rectal mucosa showed some transmural ulcers, so the colon was resected further distally and anastomosis was performed. The resected specimen showed giant cells, acute on chronic granulomatous inflammation but no hyphae. The patient was later discharged uneventfully.
Discussion
Patients with COVID-19 might present markedly increased inflammatory cytokines (such as interleukins-2, 6, 10, and tumour necrosis factor-alpha), impaired cell-mediated immunity (both CD4+ T and CD8+ T cells).1 6 Also, these patients are given high doses of steroid and broad-spectrum antibiotics.2 Hence, increased susceptibility to fungal coinfections is observed in these patients.7–9 In present case, the patient had presented with acute abdomen following COVID-19 infection and was operated after initial stabilisation. An initial possible diagnosis of ischaemic colitis, vasculitis and/or infective aetiology (tubercular or fungal) was considered. Considering the patient was on steroids due to severe COVID-19 and there has been a surge of mucormycosis cases during the present pandemic, a fungal staining of the histopathology sample was also done which confirmed our diagnosis. Previous reports have shown rhino-orbital-cerebral mucormycosis following COVID-19 infection in patient with pre-existing risk factors but intestinal mucormycosis following COVID-19 is rarely reported. Also, patient was not a known diabetic, immunocompetent and blood glucose levels were in normal range.10
Mucormycosis was first reported in humans by Paultauf (1885). The incidence of mucormycosis is reported from 0.005 to 1.7 per million population and global case fatality rate is 46%.11–13 The characteristic feature of mucormycosis is vascular invasion that results in thrombosis and tissue damage. Mostly, it is a progressive infection and can lead to death if aggressive treatment is not initiated promptly.
In a large study of 929 cases by Roden et al, commonly affected sites were sinuses (39%), lungs (24%), rhinocerebral (21%) and cutaneous (19%).3 The GI manifestations occurred in 7% cases manly involving stomach, colon and ileocecal region, probably due to ingestion of the fungus and invasion.3 It has been described mainly in premature neonates, neutropenic adults and immune-compromised patients like AIDS and organ transplant recipients.2 3 In patients with severe COVID-19 inflammation, high dose steroids and associated immunosuppression due to decreased CD4− T and CD8− T cells predispose them to fungal infections like mucormycosis.6 It may present with non-specific symptoms like abdominal pain, distention, vomiting and rectal bleeding. The patient is often misdiagnosed as having an intra-abdominal abscess3 Moreover, the fungus can invade intestinal walls and blood vessels to disseminate to other non-contiguous organs.3 4 This mortality mostly due to bowel perforation, peritonitis, sepsis and massive gastrointestinal haemorrhage.3 4 Diagnosis may be missed due to non-specific presentation and only 25% are diagnosed antemortem.4 14
Direct microscopy or Calcofluor fluorescence microscopy using a fluorescent brightening agent Calcofluor white (which binds to chitin in the fungal cell wall, and which fluoresces on excitement with UV light within 30 s) from clinical specimens such as sputum, broncho-alveolar lavage, skin lesions or histopathology is confirmatory. H&E staining was also done as part of the histology workup. Various fungi-specific stains have been used for laboratory diagnosis of the disease in clinical specimens for example, Periodic acid-Schiff, or Grocott methenamine-silver which characteristically show non-septate or pauci-septate Mucorales hyphae with a width of 6–16 lm.
Culture at 30°C and 37°C of specimens show cottony white or greyish black colony that help in the identification of species and antifungal susceptibility testing. The treatment is surgical debridement whenever possible for disease control, histopathology and/or microbiological diagnostics in addition to the systemic antifungal treatment.14 First-line antifungal monotherapy includes amphotericin B and oral suspension of posaconazole while isavuconazole can be considered for rescue treatment. In patients with high-risk factors like neutropenia and graft-versus-host disease, primary prophylaxis with posaconazole is considered.4 7 Regular radiological assessment should be followed up 6 weeks post initial imaging.11
Diagnosis of mucormycosis is difficult and timely management is essential for good outcomes. A delay of 6 days doubles 30-day mortality from 35% to 66%.12 However, despite timely diagnosis and aggressive management of mucromycosis, the prognosis is poor. Monte Junior et al had reported a case of gastric mucormycosis in a COVID-19 patient where a delay of 1 week in the diagnosis led to fatality in the patient before any surgical intervention could take place.15 In our case, the patient did not have any coexisting illness, a timely diagnosis was made and prompt treatment helped to save his life in a disease that is rare and associated with a high mortality rate.
Learning points.
COVID-19 is associated with high incidence of fungal coinfection.
High clinical suspicion for mucromycosis should be maintained in immunocompromised and COVID-19 patients.
Early identification of fungal coinfections may significantly reduce morbidity and mortality.
Aggressive management is required because of its invasiveness, potential of dissemination to other organs and poor outcome.
Pre-emptive therapy should be started if the clinical findings are suggestive of mucormycosis.
Footnotes
RPS and NG contributed equally.
Contributors: RPS did the case, helped in collecting and analysis of data, literature search, writing the manuscript critical review. NG helped in concept and design, collecting, analysis and interpretation of data, literature search, writing the manuscript and critical review. TK helped in supervision, writing the manuscript and critical review. AG helped in concept and design, collecting, analysis and interpretation of data, literature search, writing the manuscript and critical review. RS helped in writing and critical review of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Clinical management protocol for COVID-19, 2020. Available: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf [Accessed : July 7, 2020].
- 2.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005;18:556–69. 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 4.Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54 Suppl 1:S23–34. 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B. Gastrointestinal mucormycosis: an evolving disease. Gastroenterol Hepatol 2012;8:140–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from Non-COVID-19 viral pneumonia at chest CT. Radiology 2020;296:E46–54. 10.1148/radiol.2020200823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia 2020;185:599–606. 10.1007/s11046-020-00462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangneux J-P, Bougnoux M-E, Dannaoui E, Cornet M, et al. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med 2020;30:100971. 10.1016/j.mycmed.2020.100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis 2020;29:ciaa1298. 10.2139/ssrn.3644400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen M, Lahane S, Lahane TP, et al. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol 2021;69:244–52. 10.4103/ijo.IJO_3774_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019;25:26–34. 10.1016/j.cmi.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med 2021;42:264.e5–264.e8. 10.1016/j.ajem.2020.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008;47:503–9. 10.1086/590004 [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of medical mycology in cooperation with the mycoses Study Group education and research Consortium. Lancet Infect Dis 2019;19:e405–21. 10.1016/S1473-3099(19)30312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monte Junior ESdo, Santos MELD, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID-19 patient: a case report. Clin Endosc 2020;53:746–9. 10.5946/ce.2020.180 [DOI] [PMC free article] [PubMed] [Google Scholar]