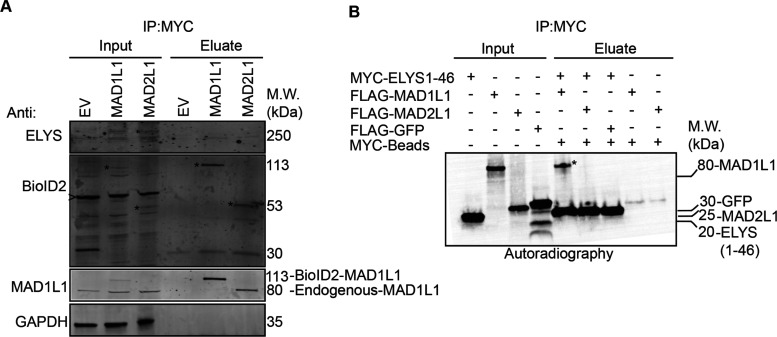
Figure 6.
ELYS binds to MAD1L1 and MAD2L1 in mitotic cell lysates and to MAD1L1 in vitro. (A) BioID2-Myc (empty vector, EV), BioID2-Myc-MAD1L1, or BioID2-Myc-MAD2L1-inducible HeLa stable cell lines were induced with Dox and treated with 100 nM Taxol to arrest cells in mitosis. Mitotic cell lysates were then used for Myc immunoprecipitations and subjected to immunoblot analysis with the indicated antibodies. Note that endogenous ELYS immunoprecipitates with BioID2-Myc-tagged MAD1L1 and MAD2L1. Asterisks indicate BioID2-Myc-MAD1L1 or BioID2-Myc-MAD2L1 in the inputs or eluates. The arrowhead indicates a nonspecific background band recognized by the anti-BioID2 antibody. (B)35S-radiolabeled Myc-ELYS N-terminal fragment (ELYS1-46, first 46 amino acids); FLAG-MAD1L1, FLAG-MAD2L1, and FLAG-GFP (control) were used in in vitro binding assays. Myc immunoprecipitations were resolved by western blotting and the blots were analyzed by autoradiography. Note that the ELYS N-terminal fragment binds to MAD1L1 (indicated by the asterisk in the eluate) and not MAD2L1.