Abstract
Background
We assessed health-related quality of life (symptoms of therapy/patient functioning/global health status), in APHINITY (pertuzumab/placebo, trastuzumab, and chemotherapy as adjuvant HER2-positive early breast cancer therapy).
Methods
Patients received 1 year/18 cycles of pertuzumab/placebo with trastuzumab and chemotherapy and completed EORTC QLQ-C30 and BR23 questionnaires until 36 months post-randomisation/disease recurrence. Changes ≥10 points from baseline were considered clinically meaningful.
Results
87–97% of patients completed questionnaires. In the pertuzumab versus placebo arms, mean decrease in physical function scores (baseline → end of taxane) was −10.7 (95% CI −11.4, −10.0) versus −10.6 (−11.4, −9.9), mean decrease in global health status was −11.2 (−12.2, −10.2) versus −10.2 (−11.1, −9.2), and mean increase in diarrhoea scores (baseline → end of taxane) was +22.3 (21.0, 23.6) versus +9.2 (8.2, 10.2). Diarrhoea scores remained elevated versus baseline in the pertuzumab arm throughout HER2-targeted treatment (week 25: +13.2; end of treatment: +12.2). Role functioning was maintained in both arms.
Conclusions
Improved invasive disease-free survival achieved by adding pertuzumab to trastuzumab and chemotherapy did not adversely affect the ability to conduct activities of daily living versus trastuzumab and chemotherapy alone. Patient-reported diarrhoea worsened during taxane therapy in both arms, persisting during HER2-targeted treatment in the pertuzumab arm.
ClinicalTrials.gov
Subject terms: Breast cancer, Breast cancer
Background
Dual HER2-targeted therapy with pertuzumab and trastuzumab significantly improves outcomes for patients with HER2-positive breast cancer in multiple settings. In patients with metastatic breast cancer, treatment with pertuzumab plus trastuzumab and docetaxel resulted in improved progression-free and overall survival compared with placebo plus trastuzumab and docetaxel.1,2 Used in the neoadjuvant setting, pertuzumab plus trastuzumab and docetaxel increased pathological complete response rates versus trastuzumab and docetaxel.3 Pathological complete response post-neoadjuvant therapy is associated with long-term clinical benefit.4 In APHINITY (NCT01358877 BIG 4–11/BO25126/TOC4939G),5 pertuzumab plus trastuzumab and chemotherapy in the adjuvant setting significantly improved 3-year invasive disease-free survival (IDFS) compared with placebo, trastuzumab, and chemotherapy in the overall population (hazard ratio [HR] 0.81, 95% confidence interval [CI] 0.66, 1.00, P = 0.045; 3-year IDFS rates: 94.1% with pertuzumab and 93.2% with placebo). The benefit appeared more marked in patients with node-positive disease (HR 0.77 [95% CI 0.62, 0.96]), and in those with hormone receptor-negative disease (HR 0.76 [95% CI 0.56, 1.04]).5 At a subsequent interim analysis, updated IDFS results further supported the clinical benefit of pertuzumab in patients with node-positive disease.6 With longer follow-up, the treatment effect was seen regardless of hormone receptor status.6 Dual HER2 blockade with pertuzumab and trastuzumab is the standard of care for first-line treatment of advanced HER2-positive breast cancer, and is also approved for neoadjuvant treatment of early breast cancer, and for adjuvant treatment of patients at high risk of recurrence, as part of a complete regimen.7,8
When selecting a treatment, the magnitude of clinical efficacy has to be balanced against the probable risk of adverse events (AEs), and effects on health-related quality of life (HRQoL). We report the results of patient-reported HRQoL measures (key secondary endpoints in APHINITY), defined as symptoms of therapy, patient functioning, and global health status.
Methods
Patients and treatment
The design of APHINITY has been described previously.5 Briefly, patients with non-metastatic, adequately excised, invasive HER2-positive breast cancer were randomised to adjuvant treatment with pertuzumab plus trastuzumab and chemotherapy, or to placebo plus trastuzumab and chemotherapy (Supplementary Fig. S1). Patients with node-negative tumours 0.5–1.0 cm were initially eligible if ≥1 high-risk feature was present. A protocol amendment was added after 3655 patients were randomised, excluding further enrolment of patients with node-negative disease. Patients could receive anthracycline- or non-anthracycline-based chemotherapy according to investigator/patient choice (non-randomised). Anthracycline-based regimens comprised three or four 3-weekly cycles of epirubicin or doxorubicin, plus cyclophosphamide (with or without 5-fluorouracil), followed by either three or four 3-weekly cycles of docetaxel or 12 once-weekly cycles of paclitaxel; or four 2-weekly (dose-dense) cycles of epirubicin or doxorubicin plus cyclophosphamide, followed by docetaxel or paclitaxel. Non-anthracycline chemotherapy comprised six 3-weekly cycles of docetaxel plus carboplatin. Pertuzumab/placebo and trastuzumab were administered intravenously: pertuzumab as an 840 mg loading dose followed by 420 mg every 3 weeks and trastuzumab as an 8 mg/kg loading dose followed by 6 mg/kg every 3 weeks. Both were initiated at the first taxane therapy cycle and continued for 1 year (maximum 18 cycles). APHINITY was conducted in accordance with the Declaration of Helsinki; all patients provided written informed consent. The protocol was approved by the appropriate ethical committee/institutional review board at each centre.
HRQoL assessments
HRQoL was defined as symptoms of therapy, patient functioning, and global health status and was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life of Cancer Patients’ Questionnaires: EORTC QLQ-C30 (core module, version 3) and its breast cancer-specific module, QLQ-BR23.
EORTC QLQ-C30 comprises 30 questions that assess five aspects of patient functioning: physical, emotional, role (ability to conduct activities of daily living), cognitive, and social; symptoms: fatigue, nausea and vomiting, pain, global health status; and additional single-items: dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties.9
QLQ-BR23 comprises five multiple-item scales that assess symptoms specific to women undergoing breast cancer treatment: systemic therapy effects, arm symptoms, breast symptoms, body image, and sexual functioning. In addition, single items assess sexual enjoyment, hair loss, and future perspective (how a patient feels about the future).10
It was recommended that a key person (e.g., research nurse) at each centre be responsible for questionnaire data collection to optimise compliance and data completeness. Patients were to complete questionnaires at the centre prior to physician assessment and before receiving study treatment at pre-specified time points: screening, the end of anthracycline therapy (if applicable), the end of taxane therapy (week 10, 13, or 19 of HER2-targeted therapy, depending on the chemotherapy regimen), week 25, the end of HER2-targeted therapy (12 months). The timing of the end of taxane patient-reported outcome (PRO) assessment was tailored to the patient’s treatment regimen to ensure that PROs were assessed at key time points, namely the end of anthracycline treatment (only in patients receiving an anthracycline-based regimen), the end of taxane therapy (all patients), and the end of HER2-targeted treatment (all patients). For patients who discontinued study treatment early, the end of HER2-targeted treatment assessment is based on the patient’s last post-baseline assessment during the treatment period, defined as up to 28 days after the last dose of study treatment. There were no clinically meaningful differences observed in functional scores between anthracycline-treated and non-anthracycline-treated patients; therefore, most analyses are shown for all patients, with the time point ‘end of anthracycline treatment’ not included. Follow-up questionnaires were to be completed by patients at 6, 12, and 24 months after therapy completion.
Questionnaires were scored according to the EORTC scoring manual; scores ranged from 0 to 100.11 In line with the scoring manual and validation papers, a pro-rated score was calculated for scales with ≥50% of the items completed. Scales with <50% of the items completed were considered missing. Absolute scores and changes from baseline were recorded, with mean, 95% CIs, median, and interquartile ranges calculated.
The mean (and 95% CIs) and median (and interquartile ranges) of the changes from baseline were calculated for patients who completed the baseline assessment, and at least one post-baseline PRO measure. A change of ≥10 points from baseline was defined as clinically meaningful based on previously published data.12 Analysis was by intention-to-treat population. To further characterise the changes in HRQoL, the number (%) of patients with a clinically meaningful worsening (reduction in score of ≥10 points) in EORTC QLQ-C30 global health status score from baseline to the end of taxane visit, end of HER2-targeted therapy visit, and the 18- and 36-month follow-up visits was calculated post hoc. The relative risk, odds ratio, and absolute risk reduction were calculated to assess differences between treatment groups. Presentation of the data in this format was limited to global health status to minimise risks associated with multiplicity.
Results
Questionnaire completion rates
Two thousand, four hundred patients were randomised to pertuzumab and 2405 to placebo. Intention-to-treat populations were 2400 and 2404 patients, respectively.5
Completion rates for EORTC QLQ-C30 and EORTC QLQ-BR23 were consistently high in both arms throughout the study, with 87–97% of patients who remained on the study at each assessment, and therefore considered evaluable, completing at least one question at that assessment (Table 1). Each scale had sufficient data for assessment. There were no notable differences in completion rates between arms or between anthracycline/non-anthracycline chemotherapies.
Table 1.
Completion rates for EORTC QLQ-C30 and EORTC QLQ-BR23.
| EORTC QLQ-C30 | EORTC QLQ-BR23 | |||
|---|---|---|---|---|
| Visit | Pertuzumab + trastuzumab + chemotherapy (n = 2400) | Placebo + trastuzumab + chemotherapy (n = 2404) | Pertuzumab + trastuzumab + chemotherapy (n = 2400) | Placebo + trastuzumab + chemotherapy (n = 2404) |
| Baseline | ||||
| Evaluable patients | 2400 | 2404 | 2400 | 2404 |
| Completed ≥1 question | 2338 (97%) | 2343 (97%) | 2337 (97%) | 2340 (97%) |
| End of taxanea | ||||
| Evaluable patients | 2239 | 2283 | 2239 | 2283 |
| Completed ≥1 question | 2120 (95%) | 2164 (95%) | 2120 (95%) | 2162 (95%) |
| Week 25 | ||||
| Evaluable patients | 2187 | 2237 | 2187 | 2237 |
| Completed ≥1 question | 2096 (96%) | 2124 (95%) | 2093 (96%) | 2124 (95%) |
| End of treatment | ||||
| Evaluable patients | 2378 | 2391 | 2378 | 2391 |
| Completed ≥1 question | 2089 (88%) | 2142 (90%) | 2088 (88%) | 2141 (90%) |
| FU month 18 | ||||
| Evaluable patients | 2208 | 2244 | 2208 | 2244 |
| Completed ≥1 question | 1960 (89%) | 1960 (87%) | 1960 (89%) | 1958 (87%) |
| FU month 24 | ||||
| Evaluable patients | 2169 | 2189 | 2169 | 2189 |
| Completed ≥1 question | 1900 (88%) | 1910 (87%) | 1900 (88%) | 1912 (87%) |
| FU month 36 | ||||
| Evaluable patients | 2094 | 2097 | 2094 | 2097 |
| Completed ≥1 question | 1859 (89%) | 1831 (87%) | 1860 (89%) | 1828 (87%) |
Evaluable patients are defined as patients still on study treatment (on-treatment visits) or still on study (follow-up period) at the expected date of the scheduled visit.
EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3, EORTC QLQ-BR23 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Breast module, FU follow-up.
aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment.
Global health status and patient functioning
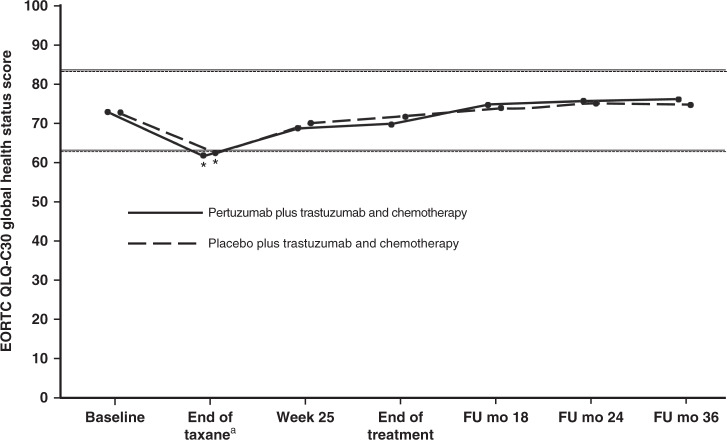
Mean global health status scores were 72.9 (standard deviation [SD] 19.7) in the pertuzumab arm and 72.5 (SD 19.7) in the placebo arm at baseline, 61.9 (SD 20.8) and 62.3 (SD 20.5) at the end of taxane treatment, 68.9 (SD 19.4) and 69.7 (SD 19.2) at the end of week 25, and 69.7 (SD 20.0) and 71.5 (SD 19.6) at the end of treatment. Scores worsened in both treatment arms up to the end of taxane treatment (as previously reported),5 and returned to baseline thereafter (Fig. 1). No clinically meaningful differences were seen at any time point in either arm. Global health status scores and changes from baseline at each visit are shown in Supplementary Table S1. At the end of taxane treatment, the percentage of patients with a clinically meaningful worsening in their global health status was 47.4% (pertuzumab arm) and 46.1% (placebo arm). Thereafter, the percentage of patients with a clinically relevant worsening fell to below 30% in both treatment arms (see Supplementary Table S2). Overall, there were minimal differences between treatment arms, as demonstrated by the 95% CI for the relative risk. The trend of the clinically relevant changes was consistent with the changes observed in the global health status mean scores, with the worst changes occurring at the end of taxane treatment, followed by an improvement thereafter.
Fig. 1. Mean EORTC QLQ-C30 global health status scores in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
Analysis is by ITT population. Higher scores indicate better health status. Horizontal lines indicate the level at which the change from baseline was considered clinically meaningful,12 with asterisks indicating time points where there was a clinically meaningful change. The lines are slightly offset to improve visibility, but time points are the same for each arm. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3; FU follow-up; mo months. From The New England Journal of Medicine, von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, and Baselga J, for the APHINITY Steering Committee and Investigators, Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer, 377, 122–131. Copyright © (2017) Massachusetts Medical Society. Adapted with permission.
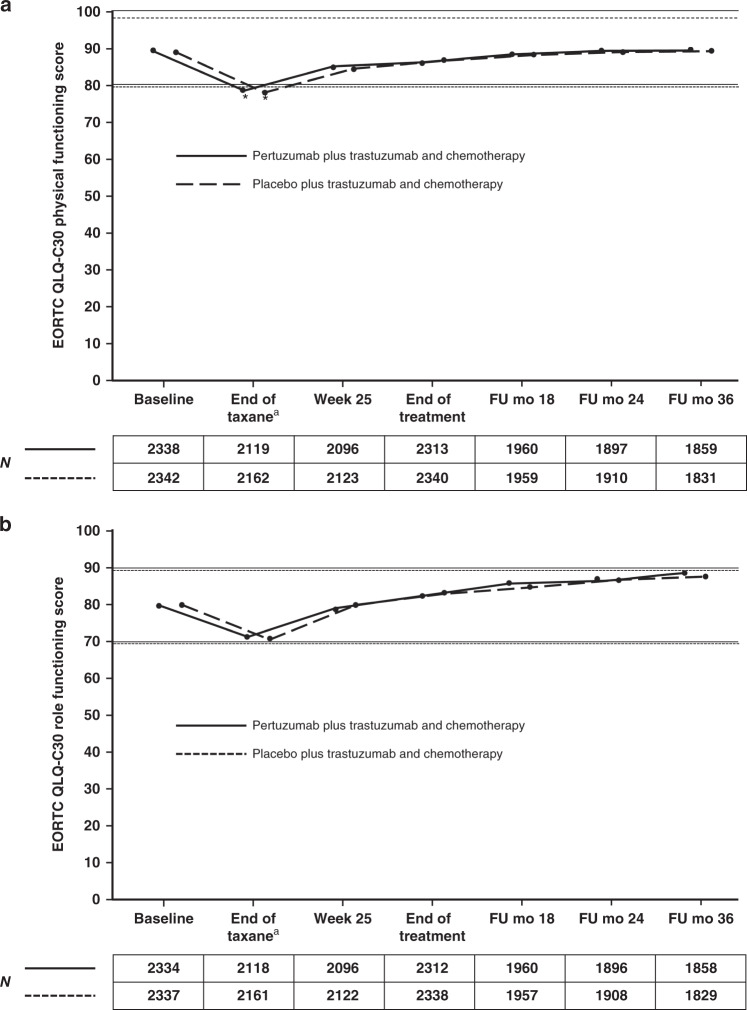
Mean scores for physical function and role function were comparable between arms throughout treatment (Fig. 2). The decline in physical function was clinically meaningful in both groups up to the end of taxane treatment (Fig. 2a; pertuzumab group −10.7, 95% CI −11.4, −10.0; placebo group −10.6, 95% CI −11.4, −9.9) and returned to baseline levels during HER2-targeted therapy. Nonetheless, patients’ ability to conduct daily activities, as assessed by role function, was maintained over the course of the study in both arms (Fig. 2b).
Fig. 2. Mean EORTC QLQ-C30 scores for functioning scales in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
a Physical functioning. b Role functioning. Higher scores indicate better health status. Horizontal lines indicate the level at which the change from baseline was considered clinically meaningful,12 with asterisks indicating time points where there was a clinically meaningful change. The lines are slightly offset to improve visibility, but time points are the same for each arm. The numbers below graphs indicate the number of patients who completed the scale. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3, FU follow-up, mo months.
Social and cognitive function were also maintained during therapy, while emotional functioning scores improved from baseline over time in both arms (Supplementary Fig. S2).
Symptoms
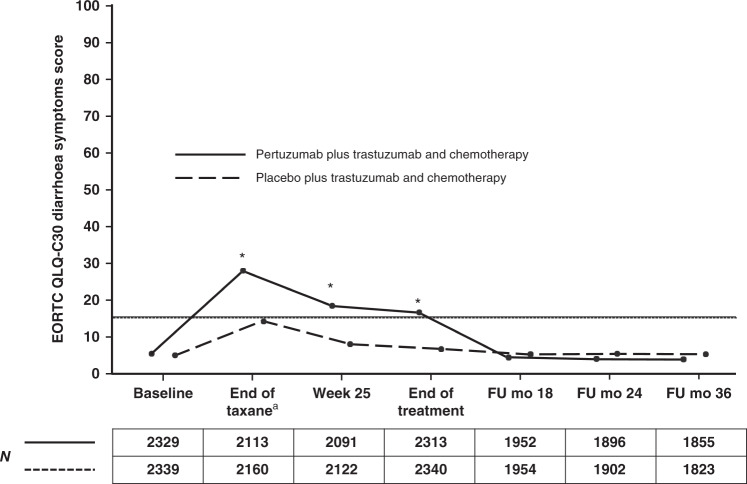
Patients in both arms reported a worsening in diarrhoea symptoms from baseline until the end of taxane treatment, with a clinically meaningful increase in the pertuzumab arm of 22.3 (95% CI 21.0, 23.6) compared with 9.2 (95% CI 8.2, 10.2) in the placebo arm. Scores improved after the end of taxane treatment but still remained elevated in both arms during the HER2-targeted treatment period. The elevation was clinically meaningful (≥10 points) in the pertuzumab group (week 25 13.2, 95% CI 12.0, 14.3; end of treatment 12.2, 95% CI 11.1, 13.4) but not the placebo group (week 25 3.3, 95% CI 2.4, 4.1; end of treatment 2.9, 95% CI 2.1, 3.8) (Fig. 3 and Supplementary Table S3). Scores in both arms returned to baseline after the end of HER2-targeted treatment. Diarrhoea symptom scores and changes from baseline at each visit are shown in Supplementary Table S3.
Fig. 3. Mean EORTC QLQ-C30 scores for the diarrhoea symptom scale in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
Numbers below graphs indicate the number of patients who completed the scale. Higher scores indicate worse/deteriorating symptoms. Horizontal lines indicate the level at which the change from baseline was considered clinically meaningful,12 with asterisks indicating time points where there was a clinically meaningful change. The lines are slightly offset to improve visibility, but time points are the same for each arm. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3, FU follow-up, mo months.
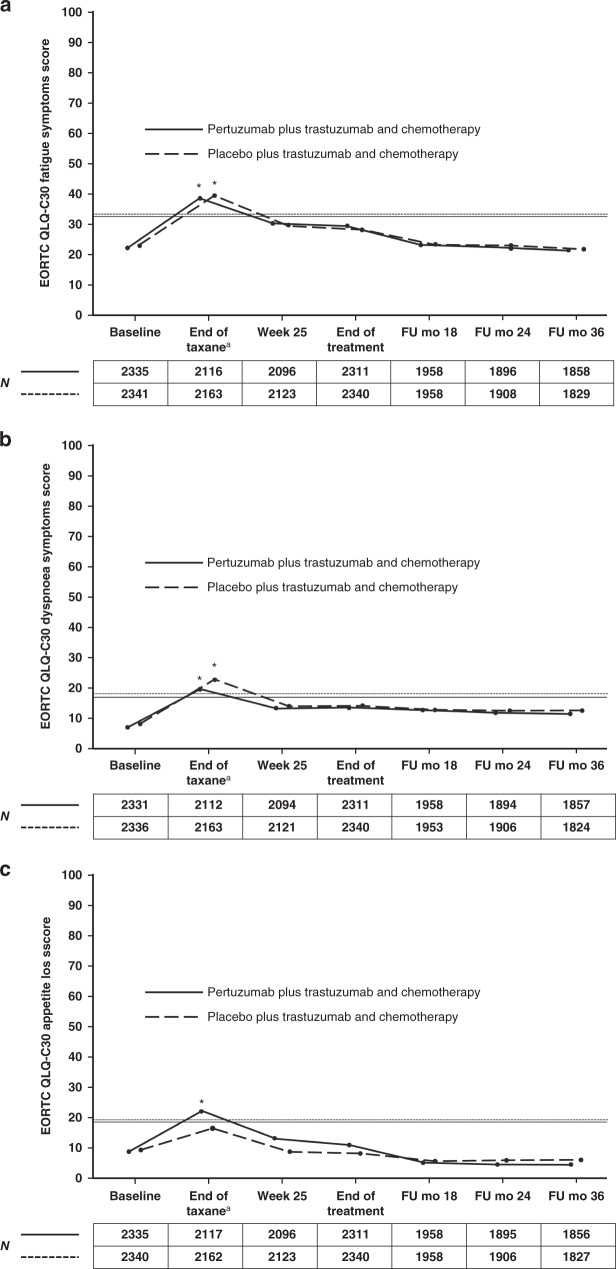
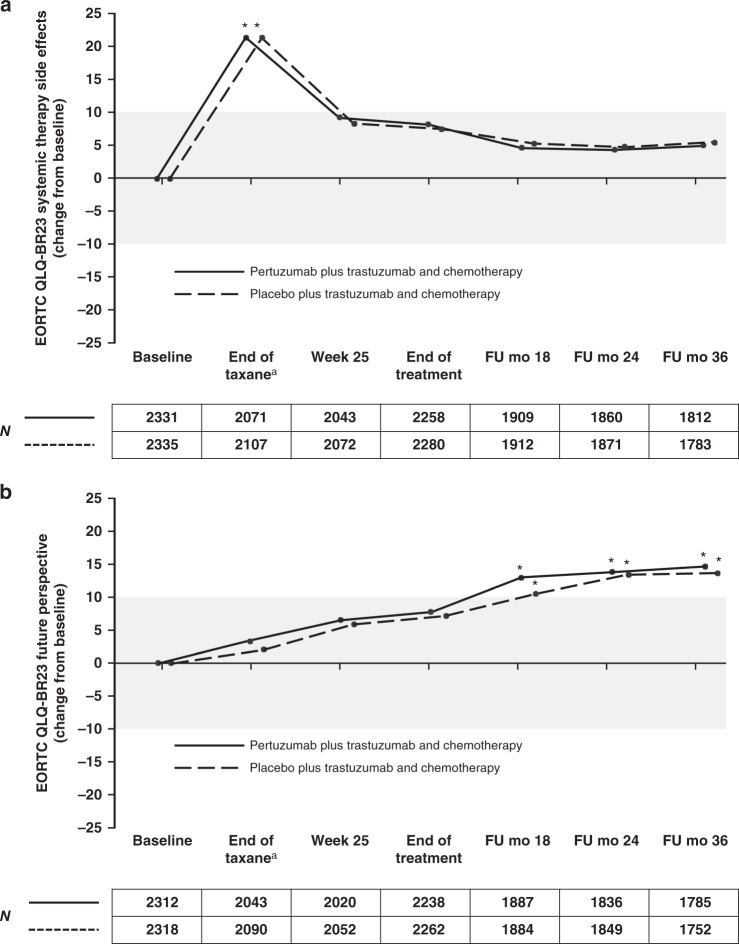
Changes in symptoms of fatigue and dyspnoea (Fig. 4a, b), systemic therapy effects (Fig. 5a), and body image (Supplementary Fig. S3a) were clinically meaningful and worsened in both arms at the end of taxane treatment, while for appetite loss the worsening of symptoms during taxane treatment was only clinically meaningful in the pertuzumab arm (Fig. 4c). During HER2-targeted therapy, the scores improved for systemic therapy side effects (Fig. 5a) and body image (Supplementary Fig. S3a) and in both arms.
Fig. 4. Mean EORTC QLQ-C30 scores for symptom scales in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
a Fatigue. b Dyspnoea. c Appetite loss. The numbers below the graphs indicate the number of patients who completed the scale. Higher scores indicate worse/deteriorating symptoms. Horizontal lines indicate the level at which the change from baseline was considered clinically meaningful,12 with asterisks indicating time points where there was a clinically meaningful change. The lines are slightly offset to improve visibility, but time points are the same for each arm. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3, FU follow-up, mo months.
Fig. 5. EORTC QLQ-BR23 scores (change from baseline) for symptom scales in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
a Systemic therapy side effects. b Future perspectives. Values outside the shaded area represent clinically meaningful changes,12 with asterisks indicating time points where there was a clinically meaningful change. Numbers below the graphs represent patients completing the response at each time point. Higher scores indicate improved future perspectives; higher scores indicate worsening in symptoms of systemic therapy side effects. The lines are slightly offset to improve visibility, but time points are the same for each arm. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-BR23 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Breast module, FU follow-up, mo months.
Scores for nausea/vomiting, pain, constipation, financial difficulties, and insomnia were maintained throughout the study period (Supplementary Fig. S4).
Scores for sexual enjoyment and upset by hair loss had clinically meaningful worsening from baseline at the end of taxane time point in both arms (Supplementary Fig. S3b, c). The scores for upset by hair loss remained worse than baseline during HER2-directed therapy, except at week 25 in the pertuzumab group. The scores improved compared with baseline for the pertuzumab group and were slightly elevated in the placebo group during the follow-up period. The sample size for this question was relatively small as only patients who experienced hair loss were asked to respond, with fewer than 300 responses at each time point during treatment and follow-up.
Scores for sexual enjoyment improved during HER2-directed therapy in both groups, but remained worse than baseline in the pertuzumab group only (mean change −10.7, 95% CI −12.6, −8.8 in the pertuzumab group; −8.0, 95% CI −9.9, −6.1 in the placebo group). The data are limited, with fewer than half of patients responding to the question. Sexual function scores are shown in Supplementary Fig. S3d.
Scores for future perspective increased during treatment and follow-up, with a clinically meaningful improvement by follow-up month 18 compared with baseline (Fig. 5b).
There were no clinically meaningful changes in the arm and breast symptom scales throughout treatment.
Function in anthracycline- and non-anthracycline-treated patients
Functioning scores for patients treated with anthracycline or non-anthracycline regimens were consistent with the overall findings, with no difference in mean scores or mean changes observed between cohorts (Supplementary Fig. S5), despite the higher incidence of diarrhoea AEs in the non-anthracycline cohort, as previously reported.13 These results should be interpreted with caution and may not be generalisable because patients were not randomly assigned between anthracycline and non-anthracycline treatment, and a relatively small proportion of patients received non-anthracycline treatment.
Discussion
Anti-cancer treatments can significantly affect patients’ HRQoL. Studies involving patients with a variety of malignancies have shown that the effect of chemotherapy and other treatments on factors such as fatigue, diarrhoea, and hair loss can also impair functional ability.9,12,14 When considering additions to a standard treatment regimen, the effect of the added treatment on HRQoL is an essential consideration when evaluating treatment benefit.12,14 Ideally, the addition of a new drug to a standard regimen should improve efficacy without causing deterioration in HRQoL.
In the curative adjuvant setting, the benefit in IDFS achieved using pertuzumab plus trastuzumab and chemotherapy needs to be balanced against any potential impact on patient HRQoL. This is particularly relevant considering the promising long-term efficacy results seen in APHINITY.6 The APHINITY trial has produced one of the largest datasets of HRQoL reported to date in patients with HER2-positive early breast cancer. Analyses of these data indicate that patients’ ability to conduct daily activities, as assessed by role function, was maintained throughout treatment and was similar in the two treatment arms. Global health status and physical function deteriorated during the taxane portion of treatment in both arms; however, the deterioration was similar, and scores returned to baseline levels after taxane therapy in both arms. Other aspects of patient functioning (social, cognitive, and emotional) were unaffected by the addition of pertuzumab to standard adjuvant therapy with trastuzumab and chemotherapy. Although there were no apparent differences between arms in worry about financial difficulties, the possible financial burden of an additional therapeutic agent does need to be considered for patients treated outside a clinical trial.
Consistent with the pattern of AE reporting, patient-reported diarrhoea worsened during taxane therapy in the APHINITY trial and remained elevated in the pertuzumab arm during HER2-targeted treatment. Diarrhoea symptoms returned to baseline levels in the pertuzumab arm after treatment completion in the follow-up phase. Most episodes were low-grade and manageable with common anti-diarrhoeals. Prompt initiation of anti-diarrhoeal treatment may improve symptom control and prevent treatment disruption.
These findings are consistent with HRQoL results from the CLEOPATRA trial, in which the addition of pertuzumab to standard trastuzumab and docetaxel in patients with metastatic HER2-positive breast cancer was assessed. PROs, as assessed by the time to clinically meaningful deterioration in the Trial Outcome Index Physical/Functional/Breast (TOI-PFB) of the Functional Assessment of Cancer Therapy-Breast (FACT-B) measure were maintained with pertuzumab therapy during treatment.15 This pattern is also consistent with the results from other studies investigating HER2-targeted agents in the early stage. In particular, in the ALTTO study, impairments of HRQoL were observed with adjuvant lapatinib, trastuzumab, or lapatinib plus trastuzumab and chemotherapy at week 12 but returned to baseline at week 52.16 Similar to APHINITY, the deterioration in HRQoL was transient and likely dominated by chemotherapy-related side effects. A deterioration in HRQoL after baseline with subsequent recovery was also described with neratinib in the extended adjuvant setting following standard adjuvant therapy (the ExteNET study); however, it was transient and during the first month of treatment, possibly linked to treatment-related diarrhoea.17 Notably, no chemotherapy was administered in ExteNET. More recent data reported from the CONTROL trial showed that proactive management decreased the rate, severity, and duration of diarrhoea, allowing patients to stay on treatment for the recommended time period.18
We utilised the threshold of a 10-point change to determine clinically important difference12 as the 10-point minimally important difference is the most commonly used threshold to assess meaningful change on the EORTC QLQ-C30 and BR23 and is referenced in the EORTC Scoring Manual.11 In light of the established literature with the EORTC scales at the time of the analysis, for comparability and consistency, the 10-point threshold was utilised in APHINITY. More contemporaneous work,19 identified small, medium, and large deterioration thresholds for each scale of the EORTC QLQ-C30. This work supports the use of the 10-point threshold used in APHINITY as many of the “medium deteriorations” are at least a 10-point change for most scales.
Drop-out of patients who did not tolerate the treatment may have affected results, although the discontinuation rate of pertuzumab/placebo due to adverse events in APHINITY was low at 7.3% in the pertuzumab arm and 6.2% in the placebo arm.5 The effect was likely therefore negligible but nevertheless, we recognise that this may be a limitation of our analysis. Most of the data are based on patients treated with an anthracycline-based chemotherapy backbone. Strengths include the above-mentioned large dataset with a high degree of questionnaire completion, and the inclusion of follow-up (post-treatment) assessments.
Conclusion
In the APHINITY trial, the addition of pertuzumab to adjuvant trastuzumab and chemotherapy improved clinical outcomes in patients with early HER2-positive breast cancer and did not adversely affect patients’ ability to conduct activities of daily living versus trastuzumab and chemotherapy alone. Patients in both arms reported a worsening in diarrhoea symptoms from baseline until the end of taxane treatment, with a clinically meaningful greater increase in the pertuzumab arm persisting throughout the HER2-targeted treatment period. Upon completion of HER2-targeted therapy, diarrhoea symptoms returned to baseline levels.
When discussing adjuvant treatment options with patients who have high-risk, HER2-positive early breast cancer, it is important to consider the side effects and patient experience with the pertuzumab, trastuzumab, and chemotherapy combination, as well as the demonstrated improvement in IDFS outcome compared with trastuzumab and chemotherapy alone.
Supplementary information
List of ethical committees and institutional review boards
Acknowledgements
The study partners: BIG, BrEAST, FSS, and F. Hoffmann-La Roche Ltd. The study sponsor: F. Hoffmann-La Roche Ltd. The Central Lab: IEO. The Steering Committee members. All members of the other committees involved in APHINITY: Translational Advisory Committee, Independent Data Monitoring Committee, Interface Committee, Cardiac Advisory Board. Support for third-party writing assistance for this manuscript, furnished by John Carron, Ph.D., of Health Interactions, was provided by F. Hoffmann-La Roche Ltd.
Author contributions
J.Bi., E.R., J.Ba., and M.Pi. contributed to the conception of the work. J.Bi., E.R., J.Ba., and M.Pi. contributed to the design of the work. E.R., A.S., D.P., J.Ba., and C.J. contributed to the acquisition of data for the work. J.Bi., J.Ba., and C.J. contributed to the analysis of data for the work. J.Bi., E.Cl., C.B., E.R., M.Pr., A.S., A.A., D.F., J.Ba., G.V., L.R., E.F., R.D.G., M.Pi., C.J., and J.A.P. contributed to interpretation data for the work. All authors contributed to drafting the work or revising it critically for important intellectual content. All authors gave final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
APHINITY was conducted in accordance with the Declaration of Helsinki; all patients provided written informed consent. The protocol was approved by the appropriate institutional review board at each centre. A full list of ethical committees and institutional review boards is listed in the supplementary material.
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: https://vivli.org. Further details on Roche’s criteria for eligible studies are available here: https://vivli.org/members/ourmembers. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Competing interests
All authors received support for third-party writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd. J.Bi. reports consulting/advisory roles for AbbVie, Genomic Health, Libbs, Lilly, Pfizer, F. Hoffmann-La Roche Ltd, and travel/accommodations/expenses from AstraZeneca and F. Hoffmann-La Roche Ltd. E.C. is an employee of Roche Products Limited and is named on issued patent ‘Uses for and article of manufacture including HER2 dimerisation inhibitor pertuzumab, 13/649591’. C.B. is a freelance pharmaceutical physician/medical advisor with Barton Oncology Ltd and has undertaken paid consultancy work with Roche Products Limited and many other companies and organisations, including (in the last ~5 years) Apeiron Biologics AG, Cancer Research UK Centre for Drug Development, Cancer Targeting Systems Inc, CellCentric Ltd, Certara LP, Innate Pharma SA, Macrophage Pharma Ltd, MorphoSys AG, Mosaic Biomedicals SL, Norgine Pharmaceuticals Ltd, Ona Therapeutics SL, Orion Clinical Services Ltd, Piqur Therapeutics AG, PTEN Research Foundation, SFL Services GmbH, T3 Pharmaceuticals AG, UCB Biopharma SPRL, and the Wellcome Trust Ltd. She is on the advisory board for SFL Services GmbH and owns shares in GlaxoSmithKline. E.R. is an employee of, and holds shares in, F. Hoffmann-La Roche Ltd. M.Pr.’s institution received funding from F. Hoffmann-La Roche Ltd with respect to the APHINITY study. A.S. reports a consulting/advisory role with Eli Lilly, Pfizer, and Novartis; travel/accommodations/expenses from Neopharm and Celgene; and has been on speaker bureaus for Teva, F. Hoffmann-La Roche Ltd, and Pfizer (all unrelated to the APHINITY study). D.F.’s and A.A.’s institution has received research funding to support the conduct of APHINITY from F. Hoffmann-La Roche Ltd/Genentech, Inc.; and research funding from AstraZeneca, Tesaro, Novartis, Pfizer, F. Hoffmann-La Roche Ltd/Genentech, Inc., and Servier, outside the submitted work. D.P. is a member of the APHINITY Joint Study Management Team and has never received any personal fees for the APHINITY study or outside of the APHINITY study from F. Hoffmann-La Roche Ltd. His institution, Institut Jules Bordet, has received both financial and non-financial (e.g., provision of drugs for study) support for the conduct of the APHINITY study. Institut Jules Bordet has received and still receives research grants or non-financial support outside of APHINITY from F. Hoffmann-La Roche Ltd. J.Ba. was an employee of AstraZeneca, served on the Board of Directors of Foghorn, and was a past board member of Varian Medical Systems, Bristol-Myers Squibb, Grail, Aura Biosciences, and Infinity Pharmaceuticals. He had performed consulting and/or advisory work for Grail, PMV Pharma, ApoGen, Juno, Lilly, Seragon, Novartis, and Northern Biologics. He had stock or other ownership interests in PMV Pharma, Grail, Juno, Varian, Foghorn, Aura, Infinity, and ApoGen, as well as Tango and Venthera, for which he is a co-founder. He had previously received honoraria or travel expenses from F. Hoffmann-La Roche Ltd, Novartis, and Lilly. He had also received from F. Hoffmann-La Roche Ltd non-financial support for studies’ drug supplies and conduct. G.V. has received travel grants and remuneration for advisory board meetings from F. Hoffmann-La Roche Ltd. His institution received investigators’ fees for the APHINITY study. L.L.R. has received airfare, taxi fare, and accommodation payments to attend APHINITY Steering Committee meetings since 2012. E.F. has received <$500 airfare and accommodation payments to attend APHINITY Steering Committee meetings. R.D.G.’s institution receives support for his salary from F. Hoffmann-La Roche Ltd, Pfizer, AstraZeneca, Merck, Ipsen, Ferring, Celgene, and Novartis. He has received travel support payments to attend APHINITY Steering Committee meetings from F. Hoffmann-La Roche Ltd. M.Pi. serves on the Scientific Board for Oncolytic. She has received honoraria for consulting roles for AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Genentech, Inc., Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Periphagen, Pfizer, F. Hoffmann-La Roche Ltd, and Seattle Genetics, and her institute has received research grants from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, F. Hoffmann-La Roche Ltd/Genentech, Inc., Servier, and Synthon. C.J. has received travel support payments for Steering Committee meetings, consulting fees, and honoraria from F. Hoffmann-La Roche Ltd for the APHINITY study. His institution received investigators’ fees for the APHINITY study. J.A.P. is an employee of Genentech, Inc. and has shares in F. Hoffmann-La Roche Ltd.
Funding
This work was supported by F. Hoffmann-La Roche Ltd, Basel, Switzerland/Genentech, Inc., South San Francisco, CA, USA (no grant number applicable). F. Hoffmann-La Roche Ltd/Genentech, Inc. was involved in the study design, data interpretation, and the decision to submit for publication in conjunction with the authors.
Consent for publication
Not required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: José Baselga.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01323-y.
References
- 1.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Roman L, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart, M., Procter, M., Fumagalli, D. de Azambuja, E., Clark, E., Ewer, M. S. et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. J. Clin. Oncol. JCO2001204 (2021). 10.1200/JCO.20.01204. Epub ahead of print. [DOI] [PubMed]
- 7.Roche Registration Ltd. Perjeta® (pertuzumab). Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/perjeta-epar-product-information_en.pdf (2019).
- 8.Genentech Inc. Perjeta™ (pertuzumab). Prescribing Information (USA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125409s123lbl.pdf (2019).
- 9.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J. Clin. Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 11.Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur. J. Cancer. 2001;37:1331–1334. doi: 10.1016/S0959-8049(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Bines, J., Procter, M., Restuccia, E., Viale, G., Zardavas, D., Suter, T. et al. Incidence and Management of Diarrhea With Adjuvant Pertuzumab and Trastuzumab in Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. Clin. Breast Cancer20, 174–181.e3 (2020). [DOI] [PubMed]
- 14.Johansson B, Brandberg Y, Hellbom M, Persson C, Petersson LM, Berglund G, et al. Health-related quality of life and distress in cancer patients: results from a large randomised study. Br. J. Cancer. 2008;99:1975–1983. doi: 10.1038/sj.bjc.6604789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortés J, Baselga J, Im YH, Im SA, Pivot X, Ross G, et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann. Oncol. 2013;24:2630–2635. doi: 10.1093/annonc/mdt274. [DOI] [PubMed] [Google Scholar]
- 16.Dueck AC, Hillman DW, Kottschade LA, Halyard MY, Sloan JA, Flickinger LM, et al. Quality of life (QOL) among patients (pts) with HER2+ breast cancer (bc) treated with adjuvant lapatinib and/or trastuzumab in the ALTTO study (BIG 2-06, Alliance N063D) J. Clin. Oncol. 2014;32(suppl):647. doi: 10.1200/jco.2014.32.15_suppl.647. [DOI] [Google Scholar]
- 17.Delaloge S, Cella D, Ye Y, Buyse M, Chan A, Barrios CH, et al. Effects of neratinib on health-related quality of life in women with HER2-positive early-stage breast cancer: longitudinal analyses from the randomized phase III ExteNET trial. Ann. Oncol. 2019;30:567–574. doi: 10.1093/annonc/mdz016. [DOI] [PubMed] [Google Scholar]
- 18.Barcenas, C. H., Hurvitz, S. A., Di Palma, J. A., Bose, R., Chien, A. J., Iannotti N., Marx G. et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann. Oncol.31, 1223–1230 (2020). [DOI] [PubMed]
- 19.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of ethical committees and institutional review boards
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: https://vivli.org. Further details on Roche’s criteria for eligible studies are available here: https://vivli.org/members/ourmembers. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.