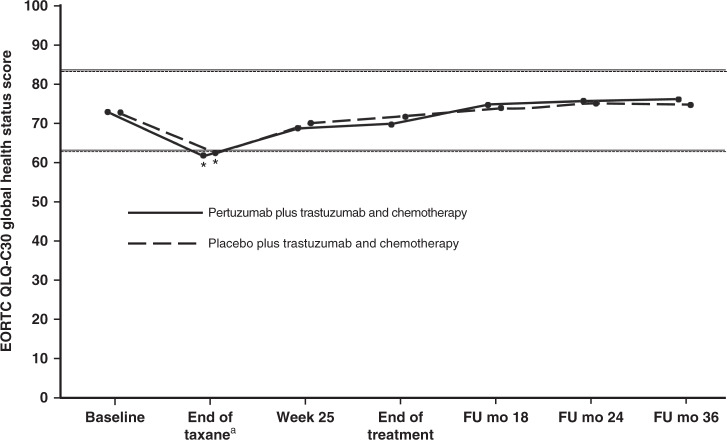
Fig. 1. Mean EORTC QLQ-C30 global health status scores in patients in the pertuzumab plus trastuzumab and chemotherapy arm (solid line) or the placebo plus trastuzumab and chemotherapy arm (dashed line).
Analysis is by ITT population. Higher scores indicate better health status. Horizontal lines indicate the level at which the change from baseline was considered clinically meaningful,12 with asterisks indicating time points where there was a clinically meaningful change. The lines are slightly offset to improve visibility, but time points are the same for each arm. aFor patients receiving anthracycline-based chemotherapy, the actual time point was week 10 or week 13 of HER2-targeted treatment (depending on the chemotherapy regimen given). For patients receiving non-anthracycline-based chemotherapy (i.e., the pertuzumab plus trastuzumab and docetaxel–carboplatin regimen), this was week 19 of HER2-targeted treatment. EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core module, version 3; FU follow-up; mo months. From The New England Journal of Medicine, von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, and Baselga J, for the APHINITY Steering Committee and Investigators, Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer, 377, 122–131. Copyright © (2017) Massachusetts Medical Society. Adapted with permission.