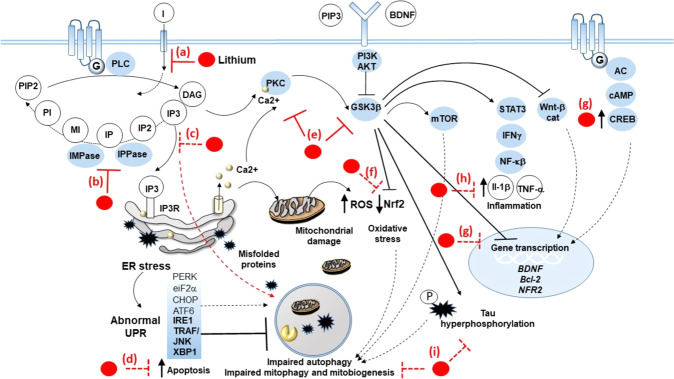
Fig. 1. Detailed drawing of the fine molecular mechanisms of lithium on specific cell targets.
Lithium inhibits the PI cycle, which is activated following stimulation of G-coupled neurotransmitter receptors (GPCRs). PLC mediates the hydrolysis of phosphotidylinositol 4,5-bisphosphate (PIP2) to the secondary messengers diacylglycerol (DAG) and inositol trisphosphate (IP3), which in turn activate downstream signaling pathways, including protein kinase C (PKC), and IP3 receptor (IP3R)/ER stress/unfolded protein response (UPR). In detail, lithium inhibits (a) the reuptake of inositol (I), as well as IMPase and IPPase (b), which results in overall depletion of IP3 (c). In this way, lithium prevents autophagy impairment and apoptosis that are bound to IP3R-related ER stress, massive Ca2+ release from the ER, abnormal UPR, as well as the accumulation of misfolded/unfolded proteins and damaged mitochondria (d). Abnormal UPR consists, for instance, of upregulation of TRAF/JNK and XBP1, which fosters apoptotic events while impairing autophagy. At the same time, through inhibition of PKC and GSK3β (e), lithium inhibits potentially deleterious effects triggered downstream of these pathways. These include (f) oxidative stress due to accumulation of reactive oxygen species (ROS) leaked from damaged mitochondria and downregulation of the nuclear factor erythroid 2 (NFE2)-related factor 2 (NRF2), which, besides its antioxidant effects, is also related to mitophagy and mitochondriogenesis; (g) impaired transcription of neurotrophic, neuroprotective, and antioxidant genes, such as BDNF, VEFG, Bcl-2, and NRF2, which is instead reinstated by lithium via both GSK3β inhibition and activation of the CREB transcription factor placed downstream of GPCRs; (h) production of pro-inflammatory cytokines underlying activation of the STAT/interferon gamma (INFγ)/nuclear factor kappa-light-chain enhancer of activated B cells (NF-kβ) pathway; and (i) accumulation of hyperphosphorylated tau, which is bound to cytoskeletal alterations and autophagy impairment.