Figure 1:
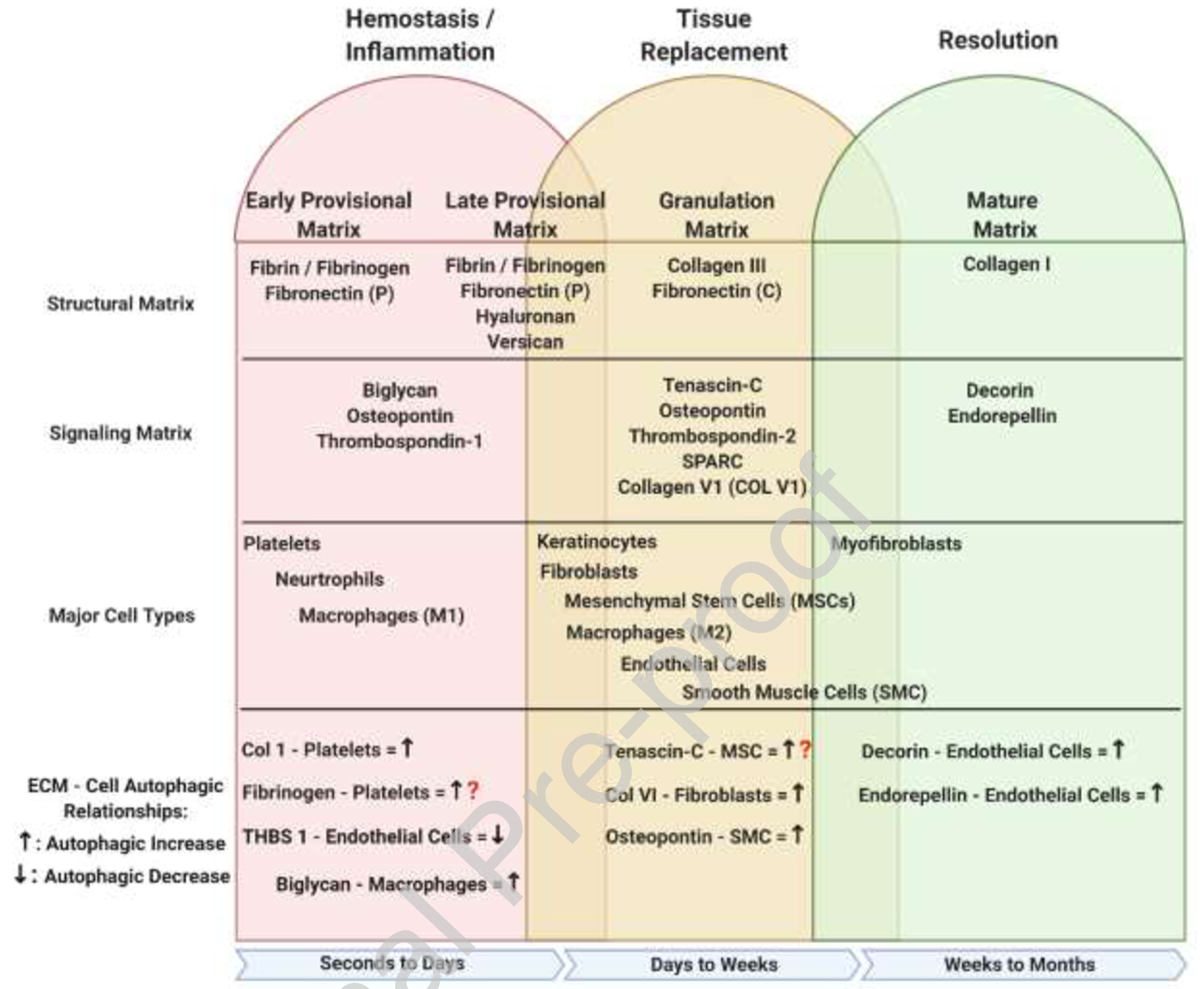
Overview of ECM-Autophagic relationships during wound healing. During the initial stages of the hemostasis/inflammation phase of repair, a provisional matrix barrier known as the fibrin clot is generated to suppress further hemorrhaging from the wound site. Concurrently with the formation of the clot, activated platelets signal to the surrounding tissue to initiate a hasty immune response recruiting neutrophils and monocyte derived M1 macrophages to sterilize the wound and remove all damaged ECM debris; this is driven by cell-specific autophagy to elicit the cell behaviors. The increase of inflammatory cell manipulations along with early fibroblasts ECM secretions further modify the provisional matrix changing it from an early to late form. The late provisional matrix further manipulates the inflammatory response, preparing it to transition from anti-inflammatory to pro-repair. During the tissue replacement phase, further modification by fibroblasts and M2 macrophages to the late provisional matrix transforms it to a granulation matrix. The granulation matrix is meant to provide easier cell movement and function as it mostly made from a loose collagen 3 base with interspersed signaling ECM proteins. The last phase of repair is the resolution phase where the ECM is recognized to its final form, vasculature is pruned, and the spare cells are removed, again involving autophagy-initiated apoptosis of endothelial cells and autophagy-triggered phenotypic shifts of fibroblasts. This figure was created with BioRender.com (on 10 October 2020). ECM – Extracellular Matrix, Fibronectin (P) – Plasma, Fibronectin (C) – Cellular, THBS1 – Thrombospondin 1, MSC – Mesenchymal Stem Cell, SMC – Smooth Muscle Cell, Col V1 – Collagen Type VI, SPARC – Secreted Protein Acidic and Cysteine Rich
