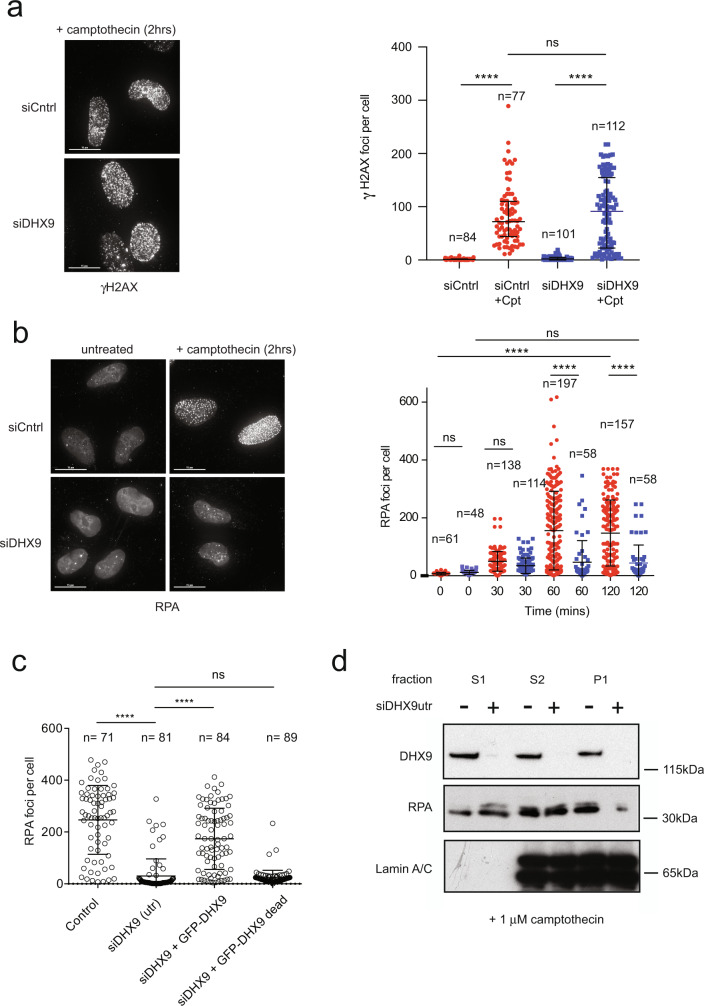
Fig. 4. DHX9 is required in DNA end-resection.
a Fluorescent image (left panel) with quantification (right panel) showing similar levels of γH2AX foci in siControl (red) and siDHX9 (blue) cells treated with 1 μM camptothecin for 2 h. b Fluorescent image (left panel) with quantification (right panel) showing formation of RPA foci in siControl (red) and siDHX9 (blue) cells treated with 1 μM camptothecin for 2 h then recovered without the drug for the indicated times. DHX9 defective cells are impaired in formation of RPA foci. c RPA foci are dependent on the helicase activity of DHX9 and are diminished in cells overexpressing siRNA resistant helicase GFP-DHX9 dead mutant. Cells were treated with 1 μM camptothecin for 2 h. Quantification of n cells (as indicated) from three pooled biologically independent experiments were performed in (a), (b), and (c). Mean and error bars for one standard deviation are shown. Data sets were shown to be significantly different using a one-way ANOVA test with Tukey’s post hoc analysis as described in Methods. (ns not significant, *p < 0.1, **p < 0.01, ***p < 0.001, and ****p < 0.0001). d Western blot showing reduced RPA in the chromatin (P1) fraction of cells treated with 1 μM camptothecin (2 h) and knocked down for DHX9. RPA in the cytoplasmic (S1) and nucleoplasm (S2) fractions is not affected by the knockdown of DHX9. Lamin A/C is shown as a control for nuclear fractionation. Source data are provided as a Source Data file.