Abstract
This study aims to assess anakinra's safety and efficacy for treating severe coronavirus disease 2019 (COVID-19). Numerous electronic databases were searched and finally 15 studies with a total of 3,530 patients, 757 in the anakinra arm, 1,685 in the control arm were included. The pooled adjusted odds ratio (OR) for mortality in the treatment arm was 0.34 (95% confidence interval [CI], 0.21 - 0.54, I2 = 48%), indicating a significant association between anakinra and mortality. A significant association was found regarding mechanical ventilation requirements in anakinra group compared to the control group OR, 0.68 (95% CI, 0.49 - 0.95, I2 = 50%). For the safety of anakinra, we evaluated thromboembolism risk and liver transaminases elevation. Thromboembolism risk was OR, 1.59 (95% CI, 0.65 - 3.91, I2 = 0%) and elevation in liver transaminases with OR was 1.35 (95% CI, 0.61 - 3.03, I2 = 76%). Both were not statistically significant over the control group. Anakinra is beneficial in lowering mortality in COVID-19 patients. However, these non-significant differences in the safety profile between the anakinra and control groups may have been the result of baseline characteristics of the intervention group, and further studies are essential in evaluating anakinra's safety profile.
Keywords: COVID-19, Anakinra, IL-1 antagonist, SARS-CoV-2, Kineret
INTRODUCTION
In December 2019, an outbreak of emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease or coronavirus disease 2019 (COVID-19) in China was brought to international notice and declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. As of January 2021, there are a total of 24,876,261 cases and 416,010 deaths attributed to COVID-19 in the United States of America [2]. The ongoing pandemic has presented a devastating effect on people's social and economic well-being by infecting over four million people worldwide. The alarmingly rising fatality rates demand an early intervention to guard and safeguard the vulnerable population. The need for innovative and effective treatment modalities is crucial as more people succumb to the virus [3]. Presently, there are no specific treatments or measures recommended to prevent or treat COVID-19. Clinical management is currently limited to supportive care [3]. Understanding the disease pathophysiology and targeting therapy towards the molecular processes is key in identifying effective therapeutic interventions. Coronaviruses are a group of RNA viruses with envelopes commonly present in mammals and birds, causing respiratory or gastrointestinal diseases with a few neurological debilities or hepatitis [4]. Infections are transmitted mainly via respiratory and fecal‐oral routes and manifest [5].
The inflammatory response in COVID-19 plays a fundamental role in the high mortality seen in patients. The virus is recognized by the innate immune system leading to the recruitment of immune cells and the production of various cytokines. Excessive production of these inflammatory cytokines causes a phenomenon termed the cytokine storm, which leads to the development and exacerbation of acute respiratory distress syndrome (ARDS) or extrapulmonary multiple organ failure. Many cytokines and chemokines have been implicated in the pathogenesis of this hyperinflammatory response [6]. This cytokine profile resembles secondary hemophagocytic lymphohistiocytosis (sHLH), a hyperinflammatory syndrome resulting in fulminant multiorgan failure. The underlying etiologies of sHLH include viral infections and sepsis [7]. The H-score is a good valid indicator of hemophagocytic syndromes including sHLH. Measurements include immunosuppression status, temperature, organomegaly, number of cytopenias, ferritin levels, triglycerides, fibrinogen, serum aspartate aminotransaminase, and hemophagocytosis features on bone marrow aspirate [8]. It has been suggested that all severe COVID-19 patients should have their H-score evaluated and used as a tool to guide therapeutics. When elevated and indicative of reactive HLH, immunosuppressives may be an option including steroids, intravenous immunoglobulin, selective cytokine inhibitors (i.e anakinra, tocilizumab) and medications inhibiting Janus kinase (JAK) pathways [7].
This systematic review and meta-analysis aims to identify the role of anakinra, an interleukin-1 receptor antagonist (IL-1Ra) in the treatment of COVID-19. Multiple studies have been performed on this drug, but the direct effects have not been reviewed in a large-scale study. We will be reviewing currently available evidence and methodology to determine the safety and efficacy of anakinra therapy in COVID-19 patients. We will analyze how anakinra modifies the disease duration and determine if it prolongs, reduces, or has no effect on viremia. Since anakinra is suspected of inhibiting a key cytokine in the pathogenesis of the hyper inflammation, the study will evaluate its therapeutic role in disease severity and secondary outcomes (i.e., the need and duration of mechanical ventilation, respiratory failure, death, etc.). Furthermore, this review will determine what dosage of the drug has shown optimal efficacy and safety in the treatment of COVID-19.
METHODS
1. Data Sources and Search Selection
This systematic review and meta-analysis was conducted based on a pre-registered protocol in ‘Prospero’ (CRD42020203708). The research was done through database searches looking for different studies to answer the clinical question to merit a systematic review and meta-analysis. A systematic review was performed according to the preferred reporting items for systematic reviews and meta-analysis guidelines (PRISMA). Institutional Review Board (IRB) approval was waived for this study because it reviews the current literature. The literature research was started on July 20, 2020 and continued for five consecutive days till July 25, 2020. Another literature search was conducted prior to the submission of the paper to include recently published articles. The research was conducted on PubMed, Medline, Cochrane Library, Google scholar, Cinahl, Clinicaltrials.gov, Embase, Scopus, medRxiv, and bioRxiv. A total of 269 articles with the potential to be included in the study were identified.
2. Study Strategy
The search for articles was done using the keywords, MeSH terms, or synonyms to collect many studies. Different keywords, MeSH terms, and synonyms including ‘COVID-19’, ‘Anakinra’, ‘IL-1 Febrile Inhibitor’, ‘Anakinra in SARS CoV 2’, ‘IL-1Ra’, ‘Urine-Derived IL1 Inhibitor’, ‘Urine Derived IL1 Inhibitor, ‘IL 1 Inhibitor’, ‘Urine’, ‘Urine IL-1 Inhibitor’, ‘Interleukin 1 Inhibitor’, ‘Urine’, ‘Antril’, ‘Kineret’, combined with Boolean operators OR/AND were used. Studies were chosen by said keywords and tabulated. After screening, the duplicate articles were removed and second-time studies that did not fulfill the inclusion criteria. At last, examinations that satisfied the qualification rules were included and affirmed through accord.
3. Inclusion & Exclusion Criteria
Primary literature was searched using the PICO design and strategy. The study incorporated all patients tested positive for COVID-19 (age >18, all sexes, all nationalities, all geographical areas, multicultural background and ethnicity), various control groups (no treatment, treatment according to the hospital guidelines, NIH guidelines), as well as the impact of using anakinra in the clinical outcome of patients (improvement, deterioration and progression to the complications) and drug efficacy. Although the research was conducted, searching for different study designs, case series, observational studies, controlled randomized and non-randomized clinical trials, published and pre-prints were included to address a significant part of gray literature and reduce the risk of selection bias.
4. Data Extraction and Risk of Bias Assessment
All data from studies were extracted by four independent reviewers onto prespecified forms. Pooled mortality was the main outcome measured. Hazard Ratio (HR) and the 95% confidence interval (CI) were extracted from each study. We selected HR as an effect estimate. We calculated the odds ratio (OR) from secondary outcomes, including the requirement for mechanical ventilation, thromboembolic events, and elevation of liver transaminases. We also extracted baseline information of the participants, sample size, and follow-up duration.
Four authors independently assessed the risk of bias qualitatively for selecting the study groups, the comparability of the groups, and the ascertainment of the outcome of cohort studies using the. The risk of bias assessment was done using the Cochrane Risk Bias Assessment and Joanna Briggs Institute Assessment (Supplementary Table 1) for the cohort studies and case series. Disagreements were resolved by discussion or with a fifth author. We evaluated the studies for the possibility of publication bias and selective reporting bias by assessing the symmetry of funnel plots visually. Due to the number of studies included, publication bias and selective outcome reporting are difficult to exclude or verify. The funnel plot's asymmetry may also indicate a difference in the methodological quality and intervention effects estimated in the studies with smaller samples.
5. Statistical Analysis
The analysis was done using Review Manager (Version 5.3, The Cochrane Collaboration, London, United Kingdom). All the outcomes reviewed were dichotomous; point estimates are expressed as OR with the 95% CI. Results were also displayed graphically by the creation of forest plots. The heterogeneity of outcomes was calculated using the I2 statistic. The I2 value of 0 - 40% was considered to be not important; 30% to 60% as moderate heterogeneity; 50 – 90% as substantial heterogeneity, and 75% to 100% as considerable heterogeneity. The meta‐analysis was performed using the Mantel Hazel method. A P-value <0.05 was considered to be statistically significant.
RESULTS
1. Literature Search Results
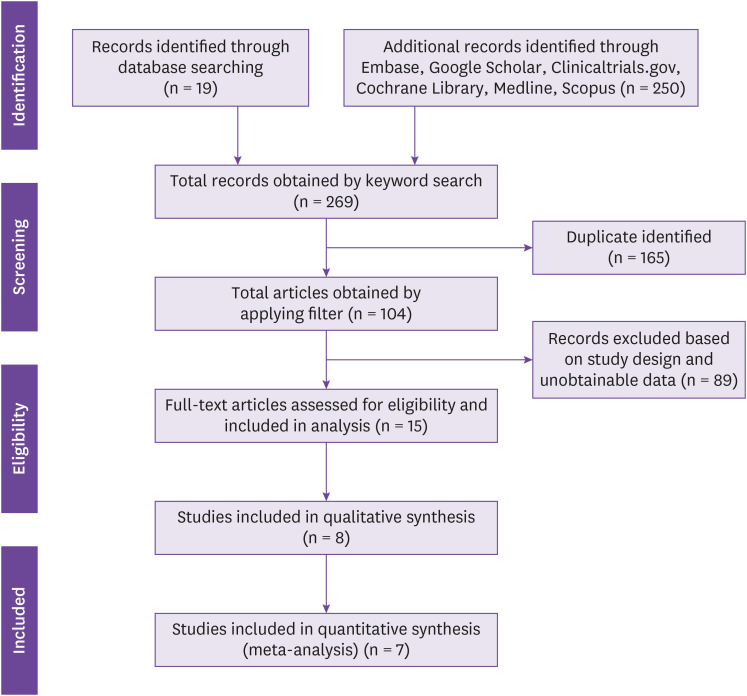
This literature search lasted for five consecutive days, utilizing various research search engines -a total of 269 articles which have been identified. The independent researchers found various studies from randomized controlled control studies, case series, case reports, and cohort studies. The PRISMA flowchart (Fig. 1) summarizes the literature search.
Figure 1. PRISMA flow diagram summarizing the literature search for included articles.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
2. Study Characteristics
The search strategy yielded 269 references after excluding 165 duplicate articles, 104 articles were subsequently screened, and 15 articles were identified for full-text review for eligibility. Among them, 5 were observational studies, 1 randomized clinical trial (RCT), 5 were case-series and 4 were case reports. The five retrospective cohort studies were designed to compare anakinra's clinical efficacy, thus were included in the meta-analysis. This meta-analysis revealed 15 articles deemed relevant for full-text review. A total of 3,530 adult patients with severe COVID-19, including 757 in the anakinra (whether administered alone or in combination with other drugs) and 1,685 in the control group arm, were included. The mean age was 55.25 years old; 12 studies had a mean age of more than 50 years old, and three studies had a mean age of less than 50 years old.
The study characteristics of the included RCT, cohort studies and case-series are available in Table 1. We encountered no multicenter studies; the five observational studies were single-center studies. The studies were conducted in different geographical locations such as France [9,10,11], Italy [12,13], and the US [14,15]. Only two studies had matched the study and control group in terms of age, sex, and disease severity. The frequency of administration ranged from once a day to four times a day with a dosage of 100 mg to 300 mg among the cohorts and the case series.
Table 1. Study characteristics of cohort studies and case series.
| Study | Authors | Study design | Country | Total sample | Mean age of treatment group | Severity of patients at admission | Intervention | Control | Dosage and Administration | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Anakinra for severe forms of COVID-19: a cohort study | Huet et al. [9] | Cohort | France | 96 | 71 | Severe COVID-19 related bilateral pneumonia, bilateral lung infiltrates, oxygen saturation 93% or less under 6 L/min of oxygen | Anakinra | Standard of care: oral hydroxychloroquine 600 mg/day, oral azithromycin 250 mg/day and parenteral beta-lactam antibiotics (ceftriaxone or amoxicillin (n = 44) | 100 mg BID for 72 hours followed by 100 mg OD for 7 days | Primary: need for ICU admission with MV or death; Secondary: death, need for MV, difference in O2 requirements, and change in CRP concentration |
| Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19 | Cauchois et al. [10] | Cohort | France | 22 | 61 | Patients presented with severe hypoxia requiring oxygen therapy between 5th to 13th day from diagnosis | Anakinra | Ritorinavir/Lopinavir/Hydroxychloroquine | Infused IV over 2 hours, OD 300 mg for 5 days, then tapered to 200 mg for 2 days, and then 100 mg for 1 day. | Mortality, oxygen requirements and days without invasive mechanical ventilation |
| Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomized controlled trial | CORIMUNO-19 collaborative group [11] | Randomized controlled trial | France | 114 | 67 | Confirmed COVID-19 with mild-to-moderate, severe or critical pneumonia, C-reactive protein serum >25 mg | Anakinra | Antibiotic drugs, antiviral drugs, corticosteroids, vasopressor support, anticoagulants | 200 mg BID on days 1 - 3 then 100 mg BID on day 4 and 100 mg OD on day 5 | Mortality and need for non-invasive or mechanical ventilation by day 4 |
| Interleukin-1blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study | Cavalli et al. [12] | Cohort | Italy | 45 | 63 | Moderate to severe ARDS and hyperinflammation (CRP >100 mg/L, ferritin >900 ng/mL), bilateral infiltrates on chest radiograph, hypoxemia | Anakinra | Standard treatment respiratory support by non-invasive ventilation with CPAP, 200 mg hydroxychloroquine BID, 400 mg lopinavir with 100 mg ritonavir BID (n = 16) | 10 mg/kg per day | Survival, mechanical ventilation-free survival, and changes in PaO2:FiO2, CRP and clinical status |
| Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study | Bozzi et al. [13] | Cohort | Italy | 120 | 62 | Ferritin >1,000 ng/mL and/or C-reactive protein >10 mg/dL and respiratory failure | Anakinra + methylprednisolone | High dose | Untreated | Mortality, blood stream infections and laboratory alterations |
| Comparative Survival Analysis of Immunomodulatory Therapy for COVID-19 'Cytokine Storm': A Retrospective Observational Cohort Study | Narain et al. [14] | Retrospective observational cohort | US | 3,098 | 65.3 | Cytokine storm (ferritin >700 ng/mL, CRP >30 mg/dL, LDH >300 U/L) | Anakinra (n = 37), Anakinra + steroids (n = 468) | Standard of care: no immunomodulatory treatment (n = 1,505) | 100 mg QID subcutaneously for 3 days then tapered | Hospital mortality |
| Use of anakinra to prevent mechanical ventilation in severe COVID-19: A case series | Navarro-Milan et al. [15] | Case series | US | 11 | 60.45 | Fever, ferritin >1,000 ng/mL, AHRF | Anakinra | N/A | 200 mg IV then 100 mg subcutaneously QID then 100 mg subcutaneously | Decreased acute hypoxic respiratory failure and prevents mechanical ventilation in patients of COVID-19 |
| Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis | Dimopolous et al. [16] | Case series | Greece and Netherlands | 8 | 67.25 | HScore >169, diffuse lung infiltrates, pO2/FiO2 <100, one patient had acute kidney injury | Anakinra | N/A | All male patients received 200 mg TID for 7 days while female patient had 300 mg | Treatment of COVID-19 patients with sHL |
| Interleukin-1 blockade with anakinra in acute leukaemia patients with severe COVID-19 pneumonia appears safe and may result in clinical improvement | Day et al. [17] | Case series | UK | 3 | 35.67 | Patient 1-case of AML, parenchymal ground glass infiltrates in the right upper lobe, increased oxygen requirement. Patient 2-case of AML, bilateral opacification of airspaces and pyrexia after starting chemotherapy. Patient 3-case of ALL, collapse and fever after 5 days of 2nd chemotherapy, lymphopenia, thrombocytopenia. | Anakinra | N/A | 2 patients 100 mg TID subcutaneously and 1 patient 200 mg BID IV | Anakinra caused clinical improvement in patients with acute leukemia and hyperinflammation |
| Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series | Aouba et al. [18] | Case series | France | 8 | 60 | COVID-19 pneumonia, CRP ≥50 mg/L | Anakinra | N/A | 100 mg BID | Blockage of cytokine storm and reduction in CRP |
| Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease | Pontali et al. [19] | Case series | Italy | 5 | 54.4 | Dyspnea associated with fever, systemic inflammation, respiratory distress, lung abnormalities on chest CT | Anakinra | N/A | 100 mg TID | Rapid resolution of systemic inflammation |
COVID-19, coronavirus disease 2019; BID, twice daily; OD, once a day; ICU, intensive care unit; MV, mechanical ventilation; CRP, C-reactive protein; IL, interleukin; IV, intravenously; CORIMUNO-ANA-1, corimuno anakinra; ANA, antinuclear antibodies; CPAP, continuous positive airway pressure; LDH, lactate dehydrogenase; QID, four times a day; AHRF, acute hypercapnic respiratory failure; N/A, not associated; TID, three times a day; sHL; secondary hemophagocytic lymphohistiocytosis; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CT, computed tomography.
3. Risk of Bias Assessment
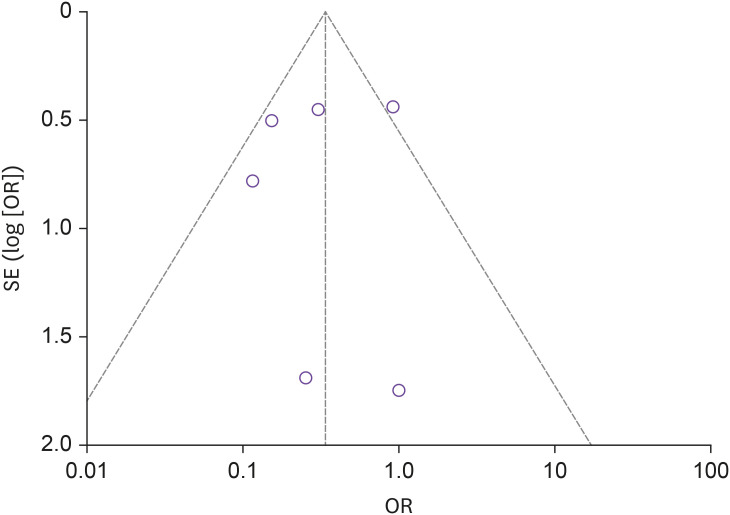
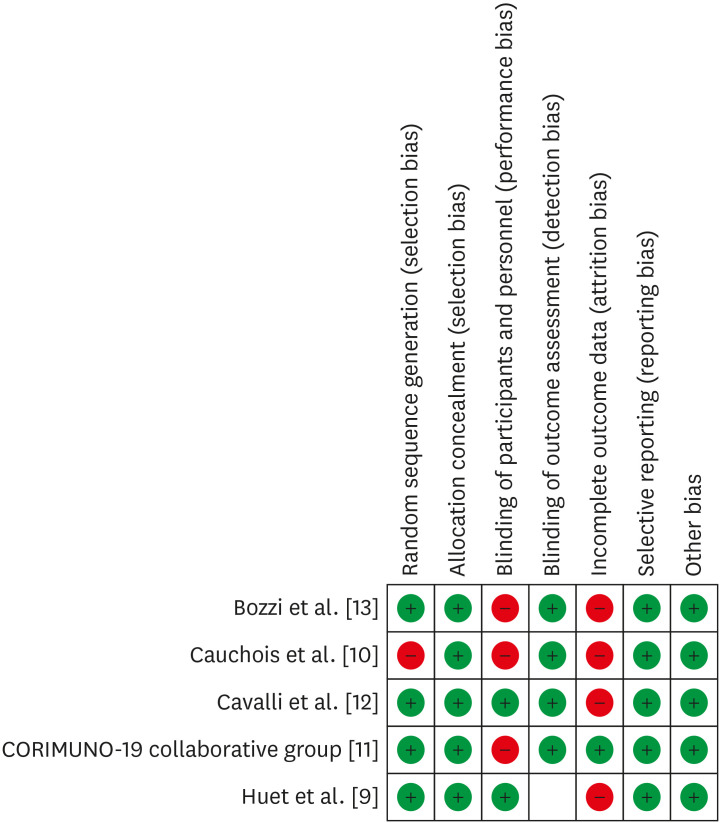
The studies overall had moderate to good methodological quality. Having a limited number of studies included in analysis (n = 7), approaches to evaluate the publication bias might have limited efficacy. However, the attempt to report the publication bias showed the funnel plot (Fig. 2) for the mortality outcome has minimal publication bias among the included studies; this could be due to sampling variability related to some studies that influences the intervention effect and its standard error. The risk of bias assessment of the cohort and RCT was done using the Cochrane Risk Bias Assessment for the included studies (Fig. 3) and Joanna Briggs Institute Appraisal Tool Risk of bias for case series (Supplementary Table 1).
Figure 2. Funnel plot showing no publication bias and high precision among studies.
The X-axis shows the drug's measured average effect and Y-axis shows standard error.
OR, odds ratio; SE, standard error.
Figure 3. Summarizing the risk of bias assessment for the included studies using Cochrane Risk Bias Assessment tool.
4. Treatment Efficacy Outcomes
1) Mortality
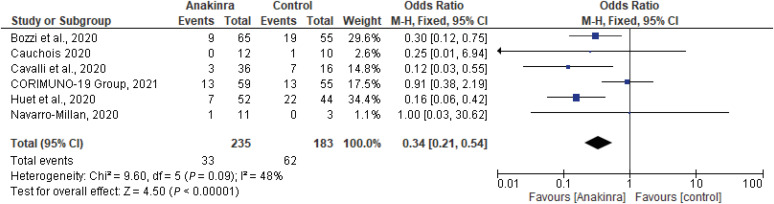
A total of six studies revealed that the all-cause mortality rate of patients with COVID-19 in the anakinra treatment group was 14.0% (33/235), which was lower compared with the control group 33.9% (62/183) [9,10,11,12,13,14]. The difference was statistically significant OR, 0.34 (95% CI 0.21 - 0.54, I2 = 48%). (Fig. 4).
Figure 4. Forest plot estimates of OR and 95% CI of the primary outcome of mortality rate between anakinra cohort studies and RCT. Summary measure reveals a statistically significant superiority in the experimental group compared to the control group P <0.000 with evidence of moderate heterogeneity I2 = 48%.
OR, odds ratio; CI, confidence interval; RCT, randomized clinical trial.
Mortality as a clinical outcome was also addressed in two case-series studies involving anakinra administration to COVID-19 patients. In case series studies by Dimopoulos et al., and Navarro-Millan et al., mortality on day 28 was estimated at 37.5% (3/8) and 9% (1/11), respectively [15,16]. No mortality was noted in the studies of Day et al., Aouba et al., and Pontali et al. [17,18,19].
2) Invasive Mechanical Ventilation
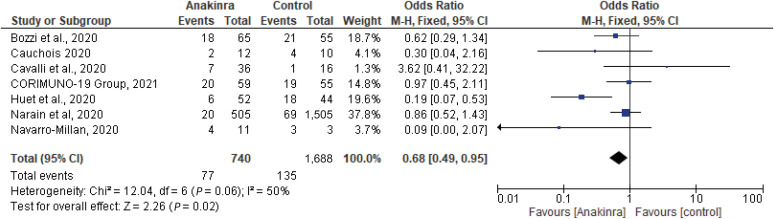
Seven studies with a total of 2,428 patients, 740 on the anakinra group and 1,688 on standard of care (SOC) were examined for the effect of anakinra on the decrease in the need for invasive mechanical ventilation (IMV) in hospitalized COVID-19 patients [9,10,11,12,13,14,15]. The pooled adjusted OR was 0.68 (95% CI, 0.49 - 0.95, I2 = 50%), indicating IMV requirement was higher in control group compared to anakinra group (Fig. 5). Forest plot for the need for IMV in the included studies showed that Huet et al. reveals a statistically significant difference favoring anakinra in survival with no need for IMV, as well as Narain et al., Bozzi et al. and Cauchois et al. [9,10,13,14]. Navarro-Millan et al. noted that early initiation of the drug before requiring supplemental O2 therapy prevented worsening in clinical status (i.e., need for mechanical ventilation). Out of four mechanically ventilated patients receiving anakinra, two patients were discharged, and one patient died [15].
Figure 5. Forest plot estimates of OR and 95% CI for the association of need for invasive mechanical ventilation and anakinra. Summary measure reveals a statistically significant superiority in the experimental group compared to the control (P = 0.02), with evidence of moderate heterogeneity I2 =50%.
OR, odds ratio; CI, confidence interval.
5. Treatment Safety Outcomes
1) Thromboembolism
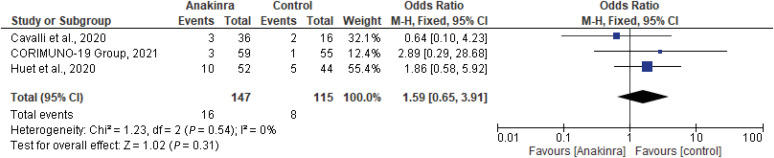
A total of three studies (two cohort studies and one RCT) with a total of 262 patients, 147 on the anakinra group and 115 on SOC were examined for the effect of anakinra and thromboembolism risk in hospitalized COVID-19 patients [9,11,12]. Safety outcome for anakinra was measured indicating 10/52 events (19.2%) in anakinra arm in Huet et al. with an OR of 1.86 (95% CI, 0.58 - 5.92), and Cavalli et al. with an OR of 0.64 (95% CI, 0.10 - 4.23) versus 5/44 events in the control group (11.3%), with no statistically significant difference between both groups (P = 0.54) [9,12]. The pooled adjusted OR was 1.59 (95% CI, 0.65 - 3.91, I2 = 0%), indicating no statistically significant association between anakinra and risk of thromboembolism (Fig. 6).
Figure 6. The forest plot depicts the estimated odds ratio and 95% CI of thromboembolic events between experimental and control groups. Summary measure reveals no statistical difference between control and experimental populations (P = 0.31), with no heterogeneity at all between pooled studies I2 =0%.
CI, confidence interval.
2) Liver transaminases
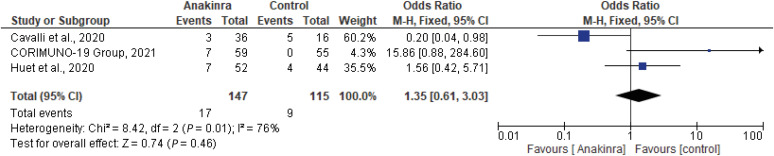
A total of three studies (two cohort studies and one RCT) with a total of 262 patients, 147 on the anakinra group and 115 on SOC were examined for the effect of anakinra and rise in the liver transaminases in hospitalized COVID-19 patients [9,11,12]. Safety outcome measurement as elevated liver transaminases in both arms showed that no statistically significant differences between both arms in Huet et al., 2020, with an OR of 1.56 (95% CI, 0.42 - 5.71), Cavalli et al., with an OR of 0.25 (0.05 - 1.25) at 95% CI, P = 0.08, and CORIMUNO-19 group with an OR of 15.86 (95% CI, 0.88 - 284.60) [9,11,12]. Aouba et al. and Navarro-Millan et al. showed no control group [15,18]. The pooled adjusted OR was 1.35 (95% CI, 0.61 - 3.03, I2 = 66%), indicating no statistically significant association between anakinra and risk of thromboembolism (Fig. 7).
Figure 7. The forest plot depicts individual and pooled estimates of odds ratios and 95% CI of increases in liver transaminases between experimental and control groups. The summary measure shows no statistical difference between experimental and control groups, (P = 0.46), with high heterogeneity presence between pooled studies I2 = 76%.
CI, confidence interval.
6. Study or Subgroup Analysis
Subgroups were created based on patient demographic data including age, gender, survival outcome, and comorbidities (Supplementary Fig. 1). A total of 2,162/4,202 in the anakinra arm (whether administered alone or in combination with other drugs), 4,845/9,940 in the control arm were included. All patients in the included studies were above 50 years of age. While accounting for gender, diabetes, and cardiovascular comorbidities, there is no difference observed between anakinra and SOC groups. Subgroup analysis of survival outcome and hypertension favor SOC with OR of 3.71 (95% CI, 1.53 - 8.97, I2 = 62%) and OR 1.36 (1.12 - 1.65, I2 = 0%), respectively. Male patients were 497 vs. 1,062 in the anakinra and control group respectively [OR 1.16 (0.96 - 1.41, P = 0.12)] while female patients were 216 vs. 605 [OR 0.87 (0.71 - 1.05, P = 0.15)]. Survival outcome Alive, No IMV) was 98/153 vs. 35/115 patients: [OR 3.71 (1.53 - 8.97, P = 0.002)], in the anakinra group and control group, respectively. Clinical comorbidities such as hypertension were found in 331/605 vs. 737/1,575 patients in the anakinra arm and control group with [OR 1.36 (1.12 - 1.65, P = 0.002)]. Diabetes is reported in 217/664 vs. 524/1,630 patients [OR 1.07 (0.83 - 1.37, P = 0.61)]. Cardiovascular comorbidities are found in 93 vs. 213 patients; [OR 0.95 (0.72 - 1.25, P = 0.70)], in the anakinra group and control group, respectively.
DISCUSSION
Respiratory failure and ARDS have been reported to be the main cause of death in COVID-19 patients followed by sepsis and multiorgan failure [20,21]. Prior to the development of ARDS, COVID-19 patients without clinical manifestations become hypoxic through preliminary endothelial damage through inflammatory cytokine release and unusually large von Willebrand factor multimers coexisting with platelet over-activation. This asymptomatic hypoxemia can be a determining feature of prognosis. Furthermore, endotheliitis through venous thromboembolism and microthrombi leads to the characteristic coagulation overactivation, coagulopathy and elevated permeability of the blood vessels due to endothelial damage in COVID-19, as seen in parallel with the H1N1 molecular pathogenesis and is also characteristic of sHLH, ARDS and multiple organ dysfunction syndrome (MODS) [21]. Delving into the molecular biology of this pathway, ARDS is primarily caused by a cytokine storm, an unregulated systemic release of inflammatory cytokines [22]. Cytokine release syndrome (CRS), also ubiquitously referred to as cytokine storm syndrome, is the process by which an elevation of inflammation and damage to the endothelium occurs and precipitates the excessive activation of the coagulation pathways as well as disrupts the integrity of the vascular permeability. A characteristic set of markers determine cytokine storm intensity, a mortality marker composed of elevated G cerebrospinal fluid, platelet-activating factor, thrombin-induced MIF, TNF, cystatin C, IL-1, IL-6, ferritin leading to immune dysregulation injury that causes endothelial damage [21,23]. CRS induced dysregulation of immune responses cause epithelial and endothelial cell apoptosis and vascular leakage, suboptimal T cell responses, dysfunctional and overactive macrophages infected with SARS-CoV-2 and dysfunctional tissue homeostasis, all of which contribute to the pathogenesis and severity of macrophage activation storm (MAS) leading to ARDS and MODS [21,22,23,24,25].
MAS is indirectly induced by IL-1 [25]. This may be the target point to prevent ARDS development by inhibition of IL-1 and resultant MAS and ARDS. Additionally, increased IL-1 release in viral infections results in lung and tissue inflammation, fever and fibrosis. An over-expression of IL-1 is pathognomonic of SARS-CoV-2 by activating transcription factor, nuclear factor, activator protein 1 and activating factor 2. This virus binds to Toll-like receptors (TLRs) to stimulate pro-IL-1 production and inflammasome activation regulating cells of both the innate and adaptive immune system and produces specific immune responses. IL1-b, thus produced, results in the inflammation of the lungs, fever and fibrosis to lead to respiratory complications in the infected host [26].
Anakinra is a recombinant, non-glycosylated form of the human interleukin-1 receptor antagonist (Recombinant human IL-1Ra [27]. It is produced by recombinant DNA technology by using an Escherichia coli bacterial expression system. It is different from native human IL-1Ra, as it has an additional single methionine residue at its amino terminus [27]. It acts by blocking the biological activity of IL-1α and IL-1β (pro-inflammatory cytokines) by competitive inhibition of IL-1 binding to the interleukin-1 type I receptor (IL-1R1). Currently, intravenous and subcutaneous routes are available with a 95% bioavailability and a half-life of 3 - 7 hours [28]. It has been used successfully in treating macrophage activation syndrome caused by various inflammatory conditions, including Still's disease, HLH, and active rheumatoid arthritis. IL-1 is produced as a result of physiological response to inflammatory stimulus and immunological conditions [29]. IL-1α and IL-1β are activated through inflammasome and pro-inflammatory cytokines that mediate many cellular responses. They also increase nitric oxide production, prostaglandin, adhesion molecules, thromboxane, and histamine [29]. These factors also contribute to the "cytokine storm" implicated in the pathogenesis of COVID-19. This mechanism of action may be helpful in COVID-19, according to previous studies. Various clinical trials are being conducted that support this rationale for targeting hyper inflammation in COVID-19 with anakinra. Outcomes are promising, but varied use and dosage and outcomes have been noticed in the various trials [27].
The impact of methylprednisolone and low dose anakinra combination therapy in patients with hyperinflammatory syndrome revealed a great statistical significance toward the treatment group in term of mortality rate as it decreases the number of deaths at day 28 by 13.9% vs. 35.6% in control group with a P-value = 0.004 and hazard ratio (HR) of 0.33 (95% CI, 0.15 - 0.47) [13]. The same effect on mortality rate demonstrated by the RECOVERY trial in dexamethasone arm for patients receiving invasive mechanical ventilation as the incidence found to be lower when compared to the control (29.3% vs. 41.4%) and rate ratio of 0.64 (95% CI, 0.51 - 0.81), while no significant impact shown on group of patients without respiratory support [30]. However, Bozzi et al. concluded a better outcome than patients in the dexamethasone group of the RECOVERY study (16.7% vs. 29.3%) [13].
Borie et al. also supported the efficacy of short term immunosuppressants in severe COVID 19 patients as it observed a lower incidence death rate (22.2%) in 180 patients treated with glucocorticoids vs. (33.3%) in 63 patients enrolled to the control group [31] but Anomar-Millan et al. was in agreement with above reported data, the 60 day mortality rate was similar between two groups received corticosteroid solely or as a combination with tocilizumab as a first line but when 10 patients received anakinra; clinical improvement noticed and that explained by a possible synergistic effects of steroid and its role in controlling the hyperinflammatory state [32].
To the best of our knowledge, this is the first meta-analysis investigating the effect of anakinra on clinical outcomes of patients with severe COVID-19 which includes an RCT. A previously published meta-analysis of non-randomized cohort studies by Pasin et al. confirm our findings. The meta-analysis included four studies with a total of 184 patients and showed significantly lower mortality and risk for mechanical ventilation in anakinra group. Furthermore, no difference in adverse events was observed between the treatment and control groups [33]. Our study includes 15 articles in both qualitative and quantitative analysis, therefore providing stronger evidence in favor of anakinra.
Based on the analysis of five retrospective studies [9,10,12,13,14], it was found that anakinra could provide additional benefit for improving the mortality outcome of severe COVID-19. However, it was found that anakinra could not provide any additional benefit for other clinical outcomes of severe COVID-19. In the study by Huet et al., of 96 patients, the mortality was 25% (13/52) compared to 73% (32/44) in control, demonstrating that overall mortality favors anakinra administration. This was further demonstrated in the study by Cavalli et al., where mortality was 10% (3/29) in the anakinra group compared to 44% (7/16) in the control group [9,12]. In a smaller size study of Dimopoulos et al. and Navarro-Millan et al., the mortality was reported to be 37.5% (3/8) and 9.09% (1/11), respectively [15,16]. In contrast, the mortality rate favored the control group in Narain et al with an HR at 95% CI 2.12 (1.68 - 2.66) [14]. In the study by Huet et al, of the 96 patients, the need for mechanical ventilation was 25% (13/52) than 73% (32/44) in control, demonstrating that the overall need for mechanical ventilation favors anakinra administration [9]. Cavalli et al noted similar findings with 17% (5/29) in the anakinra group and 6% (1/16) in the control group [12]. In a smaller size study of Dimopoulos et al. and Navarro-Millan et al., the need for mechanical ventilation was reported to be 87.5% (7/8) and 36.4% (4/11), respectively [15,16]. Several studies reported an overall decrease in the inflammatory marker levels, such as C- reactive protein (CRP) levels, ferritin levels, and NOD-like receptor protein-3 (NLRP-3) inflammasome [9,12,29,34]. Multiple other studies also supported decreased oxygen requirements at the end of the treatment period [1,3,10]. Huet et al showed that oxygen supply decreased to 2 L/min from 7 L/min [9].
Two studies noticed a significant decrease in temperature reaching normalcy in 2 - 10 days, improving oxygen requirements and respiratory function [16,17]. Two studies noticed a significant improvement in the modified WHO ordinal scale [12,15]. Cavalli et al reported improved survival in patients receiving anakinra compared to standard therapy; however, mechanical ventilation-free survival was not significantly improved in the anakinra group than the standard treatment group [12]. Navarro-Millan et al. noted that early initiation before requiring supplemental O2 therapy prevented worsening in clinical status (i.e., need for mechanical ventilation). The study also included four mechanically ventilated patients who received anakinra; two patients were discharged, and one patient died [15]. The H-score used to diagnose HLH was noted to significantly decrease at the end of the treatment period (of anakinra administration to COVID-19 patients) in the studies by Dimopoulos et al. and Day et al. [16,17]. Although both studies reported high sensitivity and specificity for diagnosing HLH, this tool has certain limitations in stratifying between moderate and severe COVID-19 patients, so severity may be underreported [35].
In our review, the therapeutic efficacy of anakinra for COVID-19 pneumonia was assessed by the need of IMV and the rate of mortality. The need for mechanical ventilation was reduced in patients treated with anakinra as compared to patients receiving SOC (OR of 0.68 [95% CI 0.49 - 0.59, P = 0.02]). However, with the therapeutic use of anakinra in patients with COVID-19 the risk of mortality was reduced by almost 20% (P <0.00001). This latter finding suggests that Anakinra is efficacious and although it may not change the course of the disease, it does increase the risk of survival. Furthermore, the utility of anakinra in the treatment of COVID-19 was found to be safe, as measured by the incidence of thromboembolic events and elevation in hepatic transaminases. There were no significant differences between these adverse events in patients treated with anakinra and those receiving SOC.
Anakinra use in COVID-19 patients has shown to have a similar safety profile with the control group without any other significant increase in adverse effects. Cavalli et al., 10 patients in the anakinra group experienced thromboembolic events than five in the control group. This was consistent in Huet et al., which showed three patients in the anakinra group than two in control having thromboembolic events. Cavalli et al., seven patients in the anakinra group had increased liver transaminase levels than four in the control group [9,12]. This was consistent in Huet et al., which showed three patients in the anakinra group than five in control [9]. Cavalli et al. identified four patients with bacteremia in the anakinra group and two in the control group [12]. Drug adverse reactions as the clinical outcome were noticed in a retrospective cohort study by Cavalli et al. in seven (24%) patients after a median treatment duration of nine days (interquartile range [IQR] 8 - 10) with high dose anakinra; four of the patients had bacteremia with the isolation of staphylococcus epidermidis and three had raised serum enzyme levels. This event led to drug discontinuation among patients with a median age of 62 years (IQR 55 - 71) with at least one comorbidity (arterial hypertension, diabetes, chronic obstructive pulmonary disease, and tobacco) [11]. No significant adverse events, including bacterial infections, were noted in the study of Huet et al. superimposed infections were most likely seen in mechanically ventilated patients due to respiratory failure [9]. Raise in procalcitonin and transaminase levels were seen in many patients. In another cohort study, raised serum liver aminotransferases were seen [9]. Other complications included injection site reaction and leukopenia in one patient. No drug allergic reactions were seen in any of the patients in the studies.
Limitations
The results of this systematic review and meta-analysis significantly favor the use of anakinra in COVID-19 patients. However, there is a risk of bias associated due to the limited number of studies. Another limitation considering the quality of the studies due to emergence of the pandemic must also be taken into consideration in which the studies were done for limited time affecting the understanding of long term consequences. All included studies except the CORIMUNO-ANA-1 trial were single-center studies which may account for publication bias in the results [11]. The factors analyzed by each study varied, therefore, also affecting the number of studies included in each of our outcome analyses. For example, mortality only included six out of our seven studies [9,10,11,12,13,14] and safety measures (thrombotic events and elevated liver transaminases) included three [9,11,12]. Our results showed that there was higher mortality in the control groups of the studies as compared to treatment groups which may be due to the inclusion criteria. Differences in the severity of COVID-19 are notable among the included studies (Table 1), hence, we must be mindful of selection bias. Furthermore, despite the pooled analysis showing favorable results for anakinra the use of other medications alongside the treatment and differences in dosage, mode and timing of anakinra administration should be taken into account. Not only were treatment protocols different but corticosteroids were not as widely used during the time of the studies, hence, standard of care was not yet established. The included RCT, however, did include corticosteroids in the control group regimen [11]. Considering the low certainty of the evidence and varied heterogeneity, it isn't easy to draw a clear conclusion in other outcomes or advantages of anakinra in COVID-19.
CONCLUSION
In conclusion, based on the current evidence, our analysis demonstrated that the use of anakinra for patients with COVID-19 was associated with the significantly low mortality rate and mechanical ventilation compared with standard care alone. Other outcomes such as thromboembolism risk and rise in liver enzymes are not significantly affected with the treatment. More extensive studies (RCTs) with long term follow-up of patients are required to provide conclusive evidence on the most effective dosing strategies and adverse effects.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: MRS.
- Data curation: MRS, MP, PH, AH, MK, RD, RV, SS, RJ.
- Formal analysis: ZD, SM, PG, NN, MN, TP, EL, CR, MS.
- Investigation: NN, MN, TP, EL, CR, MS.
- Methodology: ZD, SM, PG, NN.
- Project administration: MRS, MS.
- Resources: MK, RD, RV, SS, RJ.
- Software: MRS, MP.
- Supervision: MS.
- Validation: MS.
- Visualization: MRS.
- Writing - original draft: MRS, MP, PH, AH, MK, RD, RV, SS, RJ.
- Writing - review & editing: ZD, SM, PG, NN, MN, TP, EL, CR, MS.
SUPPLEMENTARY MATERIALS
Risk of bias for case series (Joanna Briggs Institute Appraisal Tool)
Subgroup analysis based on gender, survival outcome, comorbidities such as hypertension, diabetes and cardiovascular disease.
References
- 1.Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F, Casado JL COVID-19 ID Team. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–64. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) COVID data tracker. [Accessed 31 March 2021]. Available at: https://covid.cdc.gov/covid-data-tracker.
- 3.Centers for Disease Control and Prevention (CDC) COVID-19. Healthcare workers: Information for clinicians on investigational therapeutics for patients with COVID-19. [Accessed 31 March 2021]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- 4.Wormser GP, Rubin DH. Book review. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. Fundamental virology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2001:1408. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. Fields Virology, 4th ed. Vol. I and II. Philadelphia: Lippincott Williams & Wilkins, 2001:3280. Clin Infect Dis. 2002;34:1029–1030. [Google Scholar]
- 5.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:19. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballet JJ, Agrapart M, Durandy A, Griscelli C, Daguillard F. Separation of precursor T cells and Ig-secreting B cells from the large lymphocytic cells of human tonsil. Cell Immunol. 1977;33:291–296. doi: 10.1016/0008-8749(77)90159-9. [DOI] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 9.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bézie Y, Laplanche S, Le Berre A, Le Pavec J, Salmeron S, Emmerich J, Mourad JJ, Chatellier G, Hayem G. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, Jean R, Fouche L, Bornet C, Pauly V, Mazodier K, Pestre V, Jarrot PA, Dinarello CA, Kaplanski G. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–31. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozzi G, Mangioni D, Minoia F, Aliberti S, Grasselli G, Barbetta L, Castelli V, Palomba E, Alagna L, Lombardi A, Ungaro R, Agostoni C, Baldini M, Blasi F, Cesari M, Costantino G, Fracanzani AL, Montano N, Monzani V, Pesenti A, Peyvandi F, Sottocorno M, Muscatello A, Filocamo G, Gori A, Bandera A. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study. J Allergy Clin Immunol. 2021;147:561–566.e4. doi: 10.1016/j.jaci.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, Malhotra P, Bloom O, Liu A, Lesser ML, Hajizadeh N Northwell COVID-19 Research Consortium. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2021;159:933–948. doi: 10.1016/j.chest.2020.09.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 2020;72:1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimopoulos G, de Mast Q, Markou N, Theodorakopoulou M, Komnos A, Mouktaroudi M, Netea MG, Spyridopoulos T, Verheggen RJ, Hoogerwerf J, Lachana A, van de Veerdonk FL, Giamarellos-Bourboulis EJ. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–23.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day JW, Fox TA, Halsey R, Carpenter B, Kottaridis PD. Interleukin-1 blockade with anakinra in acute leukaemia patients with severe COVID-19 pneumonia appears safe and may result in clinical improvement. Br J Haematol. 2020;190:e80–3. doi: 10.1111/bjh.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 19.Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, Angelelli A, Caorsi R, Feasi M, Calautti F, Castagnola E, Rollandi GA, Ravelli A, Cassola G, Gattorno M. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarfraz A, Makkar S, Sarfraz Z, Hathaway D, Paul T, Sana M, Talalaev M, Perez-Fernandez J, Yatzkan G. Therapeutic plasma exchange and COVID-19: a rapid review. J Clin Immunol Immunother. 2020;6:041. [Google Scholar]
- 22.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka R, Seino KI. Macrophage activation syndrome and COVID-19. Inflamm Regen. 2020;40:19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 27.King A, Vail A, O'Leary C, Hannan C, Brough D, Patel H, Galea J, Ogungbenro K, Wright M, Pathmanaban O, Hulme S, Allan S. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2020;2:e379–81. doi: 10.1016/S2665-9913(20)30160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsevier. Transform how you use drug information. Drug Monograph – Anakinra. [Accessed 31 March 2021]. Available at: https://covid-19.elsevier.health/en-US/drug-monographs/anakinra.
- 29.Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 30.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borie R, Savale L, Dossier A, Ghosn J, Taillé C, Visseaux B, Jebreen K, Diallo A, Tesmoingt C, Morer L, Goletto T, Faucher N, Hajouji L, Neukirch C, Phillips M, Stelianides S, Bouadma L, Brosseau S, Ottaviani S, Pluvy J, Le Pluart D, Debray MP, Raynaud-Simon A, Descamps D, Khalil A, Timsit JF, Lescure FX, Descamps V, Papo T, Humbert M, Crestani B, Dieude P, Vicaut E, Zalcman G Bichat & Kremlin-Bicêtre AP-HP COVID teams. Glucocorticoids with low-dose anti-IL1 anakinra rescue in severe non-ICU COVID-19 infection: a cohort study. PLoS One. 2020;15:e0243961. doi: 10.1371/journal.pone.0243961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aomar-Millán IF, Salvatierra J, Torres-Parejo Ú, Faro-Miguez N, Callejas-Rubio JL, Ceballos-Torres Á, Cruces-Moreno MT, Gómez-Jiménez FJ, Hernández-Quero J, Anguita-Santos F. Anakinra after treatment with corticosteroids alone or with tocilizumab in patients with severe COVID-19 pneumonia and moderate hyperinflammation. A retrospective cohort study. Intern Emerg Med. 2021:1–10. doi: 10.1007/s11739-020-02600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasin L, Cavalli G, Navalesi P, Sella N, Landoni G, Yavorovskiy AG, Likhvantsev VV, Zangrillo A, Dagna L, Monti G. Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies. Eur J Intern Med. 2021;86:34–40. doi: 10.1016/j.ejim.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medscape. Drugs & Diseases. Anakinra (Rx). Brand and other names: Kineret. [Accessed 31 March 2021]. Available at: https://reference.medscape.com/drug/kineret-anakinra-343189.
- 35.Leverenz DL, Tarrant TK. Is the HScore useful in COVID-19? Lancet. 2020;395:e83. doi: 10.1016/S0140-6736(20)31057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias for case series (Joanna Briggs Institute Appraisal Tool)
Subgroup analysis based on gender, survival outcome, comorbidities such as hypertension, diabetes and cardiovascular disease.