Abstract
Despite recent advances in the management of chronic kidney disease (CKD), morbidity and mortality rates in these patients remain high. Although pressure-mediated injury is a well-recognized mechanism of disease progression in CKD, emerging data indicate that an intermediate phenotype involving chronic inflammation, oxidative stress, hypoxia, senescence, and mitochondrial dysfunction plays a key role in the etiology, progression, and pathophysiology of CKD. A variety of factors promote chronic inflammation in CKD, including oxidative stress and the adoption of a proinflammatory phenotype by resident kidney cells. Regulation of proinflammatory and anti-inflammatory factors through NF-κB– and nuclear factor, erythroid 2 like 2 (Nrf2)–mediated gene transcription, respectively, plays a critical role in the glomerular and tubular cell response to kidney injury. Chronic inflammation contributes to the decline in glomerular filtration rate (GFR) in CKD. Whereas the role of chronic inflammation in diabetic kidney disease (DKD) has been well-elucidated, there is now substantial evidence indicating unresolved inflammatory processes lead to fibrosis and eventual end-stage kidney disease (ESKD) in several other diseases, such as Alport syndrome, autosomal-dominant polycystic kidney disease (ADPKD), IgA nephropathy (IgAN), and focal segmental glomerulosclerosis (FSGS). In this review, we aim to clarify the mechanisms of chronic inflammation in the pathophysiology and disease progression across the spectrum of kidney diseases, with a focus on Nrf2.
Keywords: chronic inflammation, chronic kidney disease, mitochondrial dysfunction, Nrf2, oxidative stress, resident kidney cells
Pressure-mediated injury is a well-recognized mechanism for structural damage in CKD.1, 2, 3 Therapies that decrease intraglomerular pressure (angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers) are frequently used for the treatment of CKD and have consistent effects on pathogenic mechanisms related to blood pressure and proteinuria. Nevertheless, the effects of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers on clinically meaningful outcomes, including slowing the progressive loss of kidney function and decreasing the incidence of ESKD, are more modest.4, 5, 6, 7, 8, 9 To attenuate, arrest, or reverse CKD progression, nephrologists will need to target pathogenetic mechanisms other than altered glomerular hemodynamics.10,11
Chronic inflammation and mitochondrial dysfunction are increasingly recognized as contributing to kidney fibrosis and ESKD.12,13 Regardless of CKD etiology, chronic inflammation is likely to be present as both a cause and a consequence of glomerular and tubulointerstitial pathology.12,14, 15, 16, 17 In many forms of CKD, proteinuria is a well-recognized predictor of disease progression18 and patients with asymptomatic proteinuria exhibit low-grade inflammation linked to endothelial dysfunction.19 Whereas proteinuria contributes to the pathology of CKD by inducing adverse changes in glomerular function, such as lack of selectivity of the glomerular barrier, glomerular hypertrophy,20 and direct damage to tubule epithelial cells, it also does so by promoting chronic inflammation.21,22
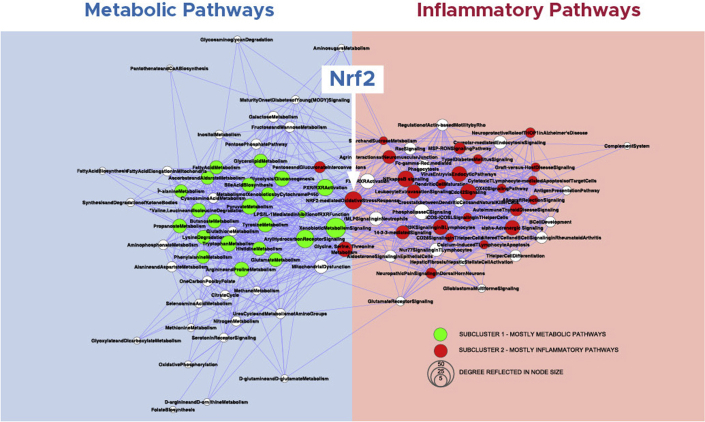
Results from a comprehensive pathway map analysis of gene sets linked to estimated GFR in 157 European patients with 9 different types of CKD indicated that inflammation and metabolism were the 2 main pathways in the pathology leading to CKD progression (Figure 1).23 Steady-state mRNA expression profiles across multiple etiologies of CKD revealed up-regulation of proinflammatory genes, including human leukocyte antigen isoforms, Toll-like receptors 1 and 3, and NF-κB1.23 This analysis revealed gene and protein interactions and defined known and novel mechanisms, which drive biological impairment across several kidney diseases, including thin basement membrane disease, FSGS, membranous nephropathy, minimal change disease, diabetic nephropathy, hypertensive nephropathy, IgAN, and lupus nephritis.23 Importantly, nuclear factor, erythroid 2 like 2 (Nrf2) anti-inflammatory pathway, discussed in detail subsequently in this review, served as a hub between 2 clusters of inflammatory and metabolic pathways activated across multiple etiologies of CKD, suggesting a common mechanism of inflammation and metabolism regulation.23 The aim of this comprehensive review is to discuss the role of inflammation and Nrf2 in the progression of CKD of different etiologies.
Figure 1.
Inflammation and metabolism in CKD progression.23 Inflammation and metabolism are 2 main pathways leading to CKD progression, with Nrf2 serving as the hub. Reproduced with permission from the American Society of Nephrology, from: Integrative biology identifies shared transcriptional networks in CKD, Martini S et al., Vol 25, Issue 11, copyright 2014; permission conveyed through Copyright Clearance Center, Inc. CKD, chronic kidney disease.
Chronic Inflammation in the Progression of CKD
Chronic Inflammation and Oxidative Stress
Persistent low-grade inflammation24 and oxidative stress (i.e., elevation of reactive oxygen species [ROS])25 are partners in crime and common hallmarks of the uremic phenotype that promotes premature aging26 and kidney fibrosis.27,28 Inflammation and oxidative stress participate in a positive feedback loop, in which each amplifies the other.29 Oxidative stress induces inflammation by activating NF-κB with the subsequent production of cytokines (e.g., interleukin [IL]-1α, IL-1β, tumor necrosis factor [TNF], IL-6)30, 31, 32 associated with the progressive decline in estimated GFR.33 Data derived from the Chronic Renal Insufficiency Cohort study revealed that circulating IL-6 and TNF receptor 2 are associated with incident CKD34 and that TNF receptor 2 is independently associated with a more rapid loss of kidney function in CKD.35
Resident Kidney Cells in Chronic Inflammation
CKD progression occurs mainly by kidney fibrosis, a process in which activated myofibroblasts are the main collagen-producing cells.36, 37, 38 Collagen, predominantly fibrillar collagens I and III, is a major contributor to fibrosis-induced cellular loss in CKD.39 Among a number of approaches that reduce experimental fibrosis, such as targeting transcription factors, signaling and developmental pathways, and epigenetic modulators, such as microRNAs, anti-inflammatory approaches have received recent attention.11 As noted previously, chronic inflammation is closely linked to both CKD initiation and progression.40, 41, 42, 43 In contrast to acute inflammation, which is a natural immune response to kidney injury44,45 that plays a role in kidney tissue repair after exposure to harmful stimuli, chronic inflammation is a maladaptive response that results from persistent stimulation of proinflammatory signaling pathways.2,3,17,44,46,47
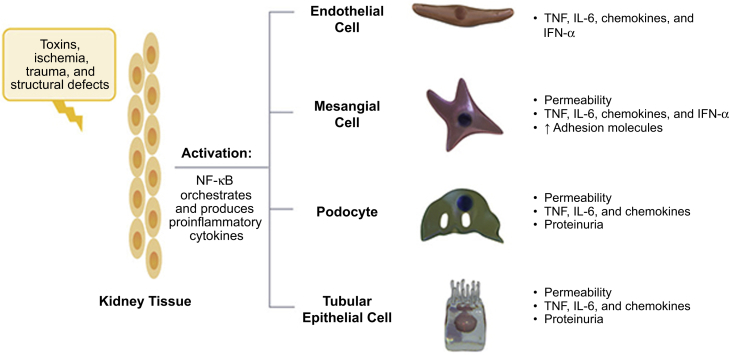
Although acute inflammation is marked by infiltrating white blood cells,48 chronic inflammation is characterized by the activation of resident kidney cells that exhibit a proinflammatory phenotype.49, 50, 51, 52, 53 Activation of resident kidney cells, including mesangial cells, endothelial cells, tubular epithelial cells, and podocytes, results in the production of proinflammatory chemokines responsible for perpetuating the cycle of chronic inflammation that eventually leads to kidney fibrosis and loss of kidney function (Figure 2).50,53,54
Figure 2.
Activation of resident kidney cells contributes to chronic inflammation in CKD.53 Resident kidney cells proliferate and produce proinflammatory chemokines responsible for perpetuating the cycle of chronic inflammation leading to kidney fibrosis. Adapted by permission from Springer Nature. Nat Rev Immunol. The immune system and kidney disease: basic concepts and clinical implications. Kurts C et al. Copyright 2013. IFN-ɑ, interferon alpha; IL-6, interleukin 6; TNF, tumor necrosis factor.
Increased levels of inflammatory cytokines and deposition of extracellular matrix (ECM) causes tubulointerstitial fibrosis, mesangial expansion, and a subsequent decline in GFR.55 On activation, mesangial cells release chemokines and cytokines, which act locally on mesangial cells, other resident glomerular cells, and leukocytes. In the presence of chronic mesangial cell activation, ECM expansion in the interstitial space causes interstitial fibrosis, which leads to glomerulosclerosis.50 In the classic 5/6 nephrectomy CKD model, NF-κB is activated and transforming growth factor beta (TGF-β) is up-regulated.56 NF-κB is a regulator of proinflammatory genes that orchestrates and produces hundreds of inflammatory cytokines and mediators.36,57,58 Activation of TGF-β causes progressive fibrosis.2 Podocytes express TGF-β after the onset of proteinuria,59 which subsequently promotes the transformation of epithelial and mesangial cells into fibroblasts and myofibroblasts.2 TGF-β also causes podocytes to produce ECM proteins that accumulate in the tubulointerstitium.59 TGF-β is synthesized by tubular epithelial cells and myofibroblasts at various stages throughout the process of kidney fibrosis.60
Proinflammatory Mediators in CKD
Glomerular damage results from the failure to eradicate harmful proinflammatory stimuli in glomerular cells or genetic mutations leading to a proinflammatory state.36,44,50 Factors that may contribute to a proinflammatory tubular cell response include release of cytokines, leakage of albumin and complement proteins, hypoxia resulting from endothelial dysfunction, and direct injury owing to immunologic, infectious, toxic, metabolic, or ischemic insults.17 Moreover, senescence of tubular cells and podocytes promoting a senescence-associated secretory phenotype with increased local secretion of inflammatory proteins links loss of kidney function to tissue inflammation.61 Kidney hypoxia/ischemia contributes to progression of kidney disease by both inflammation and oxidative stress.62 Since Nrf2 deficiency enhances susceptibility to ischemia-reperfusion–induced kidney injury,63 up-regulation of Nrf2 may protect vulnerable kidneys against repeated episodes of ischemia during adverse clinical events.
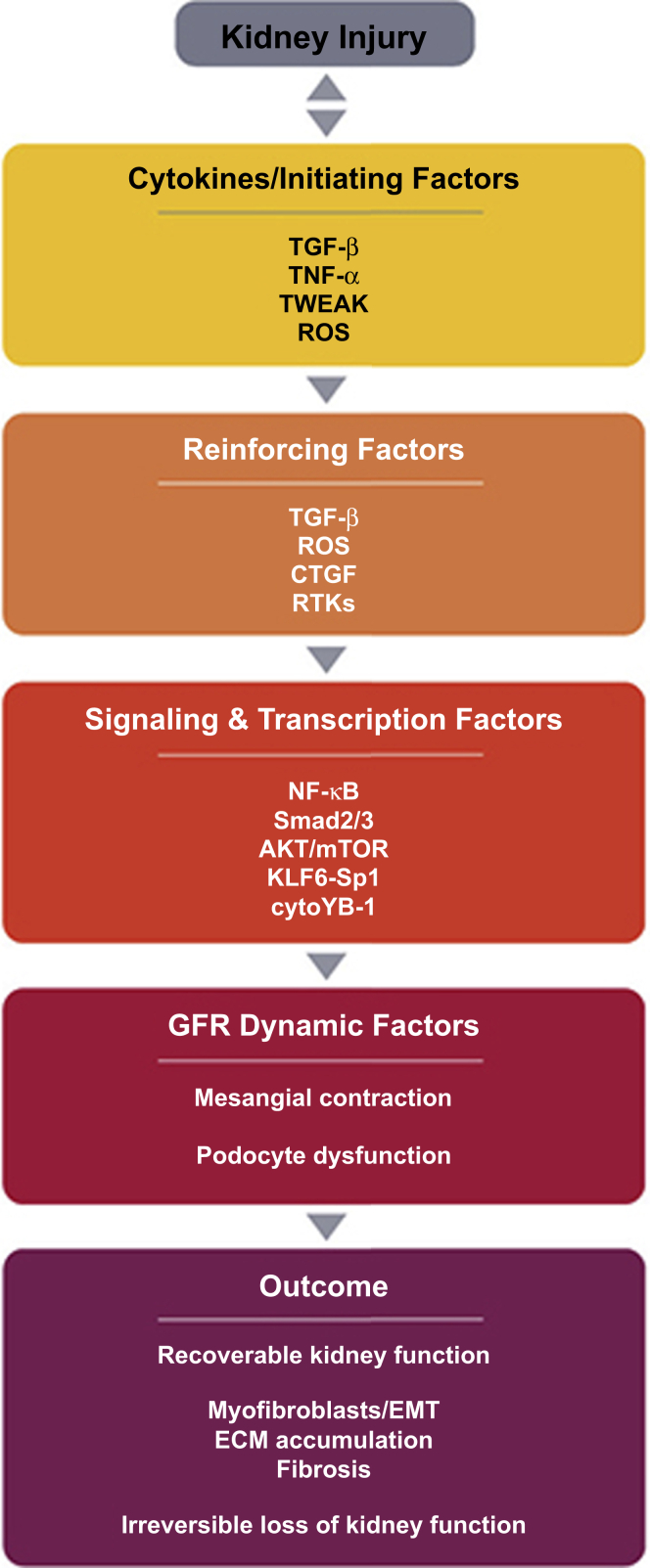
Tubular cell activation promotes further damage through interstitial leukocyte recruitment and activation, secretion of profibrotic growth factors (e.g., platelet-derived growth factor, connective tissue growth factor, and TGF-β), and stimulation of myofibroblast accumulation and activation resulting in interstitial collagen deposition and fibrosis.17,64 The multiple inflammatory mediators involved in the complex processes of chronic inflammation, remodeling, fibrosis, and loss of kidney function are summarized in Figure 3.36 Genetic and epigenetic factors are strong determinants of inflammation in CKD.23,65 One extensive analysis indicated that genotype is correlated with the presence of inflammation in patients with CKD, as defined by circulating levels of high-sensitivity CRP, whereas phenotypic features less effectively distinguish patients with inflammation from those without.66
Figure 3.
Mediators of chronic kidney disease.36 Various inflammatory mediators are involved in the complex processes leading to the loss of kidney function. Adapted with permission from Elsevier. Originally published in Eur J Pharmacol. Vol 820. Lv W et al. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Pages 65 to 76. Copyright 2018, Elsevier. ECM, extracellular matrix; EMT, epithelial-mesenchymal transdifferentiation; GFR, glomerular filtration rate; ROS, reactive oxygen species; TNF, tumor necrosis factor; TGF-β, transforming growth factor beta; TWEAK, TNF-like weak inducer of apoptosis.
Proinflammatory Role of NF-κB in CKD
In CKD, the activation of ROS-generating enzymes (e.g., nicotinamide adenine dinucleotide phosphate oxidase and xanthine oxidase) contributes to elevated oxidative stress in the kidney.67 Oxidative stress triggers activation of NF-κB, enhancing the inflammatory response. NF-κB orchestrates and stimulates the production of a variety of inflammatory cytokines and mediators.2,36,57,58 Cytokines produced from many resident kidney cells recruit macrophages to damaged tissue, which results in increased production of proinflammatory factors and progressive glomerulosclerosis.2,3
Anti-inflammatory Role of Nrf2 in CKD
Nrf2 represents a critical cellular factor that evolved as a protection against oxidative stress when organisms hundreds of millions of years ago began to explore the world above the oceans and were exposed to oxygen.68, 69, 70, 71 A member of the bZIP family of transcription factors—specifically in the CNC subfamily—Nrf2 has 7 structural domains (Neh1 to Neh7) with varying functions. Nrf2 heterodimerizes with sMaf proteins K, G, and F within the nucleus, which facilitates recognition of an enhancer sequence known as an antioxidant response element that is found in the regulatory regions of more than 250 genes.3,70,72, 73, 74
The Role of Nrf2 in Regulating Inflammation
Nrf2 protects kidney cells and other tissues by upregulating an array of genes and consequently attenuating the production of proinflammatory cytokines.15 Molecules with levels that are increased by Nrf2 include catalase, superoxide dismutase, glutathione peroxidase, heme oxygenase-1, reduced nicotinamide adenine dinucleotide phosphate quinone oxidoreductase, and glutamate-cysteine ligase.15 An equilibrium between protein synthesis and proteasomal degradation is required to maintain the intracellular concentration of Nrf2 at a low level.74 The cytosolic inhibitor Keap1 binds to Nrf2 in the cytoplasm.67,71,75
Under normal conditions, Keap1 targets Nrf2 for degradation by the ubiquitin-proteasome system (Supplementary Figure S1).55,76 Under conditions of oxidative stress, electrophiles and ROS alter the conformation of Keap1 by forming direct adducts with specific sensor cysteine residues.55,76 Such modifications alter the interaction between Keap1 and Nrf2, resulting in decreased Nrf2 degradation in cells exposed to oxidative stress. Nrf2 then translocates into the nucleus where it activates the transcription of its target genes.
Nrf2 also directly suppresses the expression of proinflammatory NF-κB target genes by binding to their promoters and inhibiting transcription.77 The substantial crosstalk between Nrf2 and NF-κB pathways controls the expression of multiple downstream target genes.78 Thus, the Keap1-Nrf2 system plays a key role in the resolution phase of inflammation by opposing oxidative damage and through inhibition of proinflammatory NF-κB signaling.72,79,80
Evidence from the animal kingdom suggests that Nrf2-based antioxidant defense mechanisms have evolved to protect species during extreme conditions.68 Inhibition of Nrf2 activity promotes stress-induced premature senescent phenotype81 and Nrf2 expression decreases with aging in mice.82 Based on recent findings showing that down-regulation of Nrf2 activity promoted oxidative stress and accelerated cellular senescence, it was suggested that drugs targeting Nrf2 signaling could suppress cellular senescence-associated pathologies.83 Since the activity of Nrf2 is reduced in Hutchinson-Gilford progeria syndrome, a rare syndrome of premature aging,84 and a study of Nrf2 knockout mice stressed by space travel showed increased generation of age-associated metabolites,85 Nrf2 may play a protective role in aging processes. Indeed, persistent low-grade inflammation (i.e., “inflammaging”) and decreased Nrf2 expression are prominent features of CKD and other burden-of-lifestyle diseases associated with premature aging.68 Kidney fibrosis and epithelial-mesenchymal transition—a process in which differentiated epithelial cells undergo a phenotypic conversion that generates matrix-producing fibroblasts and myofibroblasts—contribute to aging in the kidney86 and may reflect a premature aging process confined to the kidney tissue.11 As other organs undergo premature aging processes in the inflamed uremic milieu, progression of kidney disease due to fibrosis and inflammation often associate with the parallel development of a uremic phenotype characterized by vascular calcification, sarcopenia, osteoporosis, etc (Supplementary Figure S2).87
The Role of Nrf2 in Kidney Disease
Results from multiple studies highlight the critical role of Nrf2 in kidney disease.88, 89, 90, 91 A recent systematic review of 32 studies concludes that whereas Nrf2 expression was consistently downregulated in CKD, NQO1, and HO-1 showed varying alterations related to inflammation, comorbidities, and severity of kidney damage.92 Jiang et al.69 showed that kidney biopsy specimens from patients with DKD demonstrated high levels of glucose-induced ROS in mesangial cells as well as activation of Nrf2 and downstream genes. By immunohistochemistry, it was demonstrated that whereas Nrf2 was expressed at low levels in normal glomeruli, it was up-regulated in glomeruli from patients with DKD.69 In contrast, an analysis of 20 patients on hemodialysis, 20 additional patients with nondialysis-requiring CKD, and 11 healthy individuals that included evaluation of Nrf2 and NF-κB expression with real-time polymerase chain reaction showed that Nrf2 gene expression was reduced and NF-κB expression increased in peripheral blood mononuclear cells from patients on hemodialysis compared with those from healthy individuals and patients with nondialysis-requiring CKD.93
Animal experiments also demonstrate a key role for Nrf2 in controlling uremic inflammation. Nrf2-knockout mice administered streptozotocin have higher ROS production and more pronounced oxidative DNA damage and kidney injury vs similarly treated wild-type animals.69 Impaired Nrf2 activation has also been associated with kidney fibrosis and disease progression in a mouse model of focal glomerulosclerosis.94 In accordance, impaired Nrf2 signaling and NF-κB activation promote inflammation and oxidative stress in a mouse remnant kidney model.15,95
Nrf2 activation also prevents or attenuates fibrosis. Unilateral ureteral obstruction in mice results in the down-regulation of Keap1, allowing for the rapid accumulation of Nrf2 in the nucleus and induction of Nrf2-dependent gene expression, which prevents generation of ROS. However, longer-term obstruction leads to a progressive reduction in nuclear Nrf2 as well as reduced levels of antioxidants and increased oxidative stress, inflammation, fibrosis, and tubular damage.96 These studies reveal the potential variability of Nrf2 expression in CKD based on disease progression since Nrf2 may be up-regulated in early stages due to ROS but can be downregulated as the disease worsens and inflammation is exacerbated.
Sodium-glucose cotransporter 2 inhibitors—a new class of renoprotectors—have been found to promote a substantial reduction in albuminuria and a reduced risk of progression to ESKD in both type 2 diabetic and nondiabetic CKD.97 As part of the renoprotective effects of sodium-glucose cotransporter 2 inhibitors seems to be mediated by improvement of renal hypoxia98 accompanied with reduced inflammation99 and improvement of antioxidant defense expression,100 it is of interest that it was recently reported that dapagliflozin restrains apoptosis and activates autophagy in part by activation of Nrf2/HO-1 pathways.101
Mitochondrial Dysfunction and Nrf2 in the Progression of CKD
Accumulating evidence suggests that mitochondrial dysfunction promotes the development and progression of CKD irrespective of the underlying cause.13 Mitochondria are complex organelles with various functions, including the generation of adenosine triphosphate by oxidative phosphorylation.102
More recently, our understanding of the roles mitochondria play has expanded beyond adenosine triphosphate production to include an appreciation of their function as organelles that regulate cellular processes, such as proliferation, differentiation, and death.103 Mitochondria act as a central hub within the cell, sensing changes in the cellular milieu and rapidly redirecting metabolic intermediates to appropriately meet the demands placed on the cell.104 It is now apparent that metabolic reprogramming plays a critical role in the inflammatory response.105 Encountering a pathogen triggers a phenotypic switch in macrophages that is characterized by a decrease in the rate of oxidative phosphorylation and fatty acid oxidation and a robust increase in the rate of glycolysis, lipid synthesis, and ROS production.106,107 Although ROS are necessary to mount both an innate and an adaptive immune response against a variety of invaders, such as bacteria,103 their overproduction leads to oxidative stress resulting in tissue damage and dysfunction.30 This metabolic reprogramming is meant to be short-lived and restricted to the locale of infection or damage. Once the stress has been eliminated, it is critical that cells return to homeostasis—a balanced state where inflammatory processes are turned off, ROS are neutralized, and mitochondrial metabolism reverts to a normal state of oxidative phosphorylation.108 In CKD, however, this “off switch” fails and the damaging processes are sustained, ultimately leading to tissue damage and loss of kidney function.44,73
Nrf2 is known to reduce ROS levels and suppress inflammation; however, recent studies have found that this transcription factor also regulates cellular and mitochondrial metabolism.30,109, 110, 111, 112 Nrf2 directs metabolic reprogramming by regulating glucose and lipid metabolism, increasing efficient adenosine triphosphate production, promoting mitophagy, and increasing mitochondrial biogenesis.112,113 By suppressing inflammation, reducing ROS levels, and supporting the structural and functional integrity of mitochondria, Nrf2 improves the ability of the cell to recover from cellular stress and restores cellular homeostasis.109,112,113
The kidneys are highly metabolic organs that require large amounts of adenosine triphosphate to function normally.102 Owing to their high oxygen consumption, the kidneys are susceptible to damage caused by ROS, which can accelerate kidney disease progression.13,114 Overproduction of ROS and defective mitophagy, along with activation of apoptotic pathways, which are regulated by mitochondria, are interconnected drivers of CKD progression.13 Mitochondrial dysfunction in kidney cells seems to be implicated in the risk of kidney disease. Higher mitochondrial DNA copy number, a surrogate marker of mitochondrial function improvement, has been associated with a lower risk of CKD, independent of traditional risk factors and inflammation.115 In contrast, reduced expression of mitochondrial-derived peptides has been associated with inflammation and reduced expression of Nrf2 in CKD.116 Furthermore, mitochondrial dysfunction is believed to play a role in kidney fibrosis117 and the progression of kidney disease.108 Taken together, these findings suggest that these metabolic powerhouses may be a therapeutic target to attenuate progression of kidney disease.118 A recent study revealed that an analog of bardoxolone methyl, a potent activator of Nrf2, confers protection from proteinuria-induced mitochondrial damage to tubules both in vitro and in an animal model by improved mitochondrial redox balance and mitochondrial function.21
Chronic Inflammation Across the Spectrum of Kidney Disease
Alport Syndrome
Chronic inflammation in Alport syndrome, similar to other conditions resulting in CKD, is a result of persistent activation of proinflammatory signaling pathways.2,3,17,44, 45, 46, 47 Activated macrophages have been found to contribute to disease progression in Alport syndrome.2 COL4A3 knockout mice develop proteinuria as early as 5.5 weeks of age.119 Excessive protein in the glomerular filtrate (“pathological proteinuria”) activates proinflammatory and profibrotic signaling pathways in proximal tubular epithelial cells2 and leads to the expression of genes encoding chemotactic molecules. These molecules promote infiltration of immune cells, leading to tubular atrophy and tubulointerstitial fibrosis.2 TGF-β is also involved in the processes of glomerulopathy and fibrosis.2,59,120,121 As patients with Alport syndrome experience a loss of kidney function despite the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and there is a documented inflammatory component, the effects of Nrf2 stimulation with bardoxolone methyl are currently investigated in a randomized controlled trial (CARDINAL).122
Autosomal-Dominant Polycystic Kidney Disease
Inflammation is evident early during the course of ADPKD when patients have normal or near-normal kidney function. Results from several studies support a role for inflammation in ADPKD cyst development and disease progression. Cyst formation has been found to precede interstitial macrophage accumulation suggesting these cells are migrating to sites of inflammation.123,124 Regulation of cyst growth by macrophage migration inhibitory factor provides further support for inflammation as a stimulating factor in cyst development and for the involvement of macrophages in ADPKD.125 Animal studies also support a significant role for inflammation in the development and progression of ADPKD. For example, macrophage depletion in polycystin 1, a transient receptor potential channel interacting (Pkd) 1-targeted PKD mouse, resulted in a less severe cystic phenotype and better kidney function.126 NF-κB has been detected in the nuclei of cyst-lining cells in a mouse PKD model,127 and elevated levels of proinflammatory cytokines, including IL-1β, TNF, and IL-2, have been reported in ADPKD.128,129 These observations indicate that chronic inflammation may not only promote the initiation of disease and cystogenesis through its cellular effectors but also play a role in cyst expansion and disease progression.123,125 Indeed, it was recently reported that activation of Nrf2 ameliorates oxidative stress and cystogenesis in a mouse model of ADPKD.91
IgA Nephropathy
Inflammation in IgAN results from immune complex formation of galactose-deficient IgA1 in the glomerular mesangium.130 Immune complex formation arises subsequent to multiple sequential immunopathogenic “hits,”131, 132, 133 including induction of local inflammatory responses, IgA deposition in glomeruli, and activation of—and damage to—mesangial cells. Activated mesangial cells secrete components of ECM and release multiple mediators that contribute to kidney injury, including proinflammatory and profibrotic cytokines,134 which stimulate mesangial cell proliferation and recruitment of inflammatory cells into the glomerulus.132 Inflammatory mediators also modify gene expression in podocytes, resulting in podocyte injury (“glomerulopodocytic crosstalk”) and filtration of IgA immune complexes, including segmental glomerulosclerosis.132,135, 136, 137, 138 An animal study has suggested that stimulation of the Nrf2 pathway has the potential to modulate inflammation in IgAN. In mice with induced accelerated and progressive IgAN, stimulation of the Nrf2 pathway with antroquinonol inhibited T cell activation and prevented activation of the NLR family pyrin domain containing 3 inflammasome. It also significantly improved proteinuria, kidney function, and histopathology in accelerated and progressive-IgAN mice with established disease.139
Diabetic CKD
Proinflammatory signaling pathways and their downstream products are emerging as new biomarkers in DKD and may be promising therapeutic targets in patients with this disease.140 DKD involves activation of chronic inflammatory pathways that contribute to disease progression141 and is associated with multiple inflammatory cell types, molecules, and pathways, including macrophages, mast cells, and NF-κB–mediated transcription of inflammatory cytokines, including IL-1, IL-6, IL-18, and TNF.141, 142, 143, 144, 145 A signature of circulating inflammatory proteins enriched in the TNF receptor superfamily members predicted the 10-year risk of ESKD in diabetes,146 suggesting drugs targeting inflammation could help arrest progression of DKD. Ultrastructural changes in the glomerular basement membrane result from the presence of acute-phase markers of inflammation, such as IL-6.147 NF-κB–induced molecules and pathways result in structural alterations and functional abnormalities characteristic of DKD, and ultimately, kidney failure in these patients.141 A role for Nrf2 in DKD was found in a study of diabetic rats revealing impaired kidney function was correlated with oxidative stress and reduced translocation of Nrf2 into the nucleus.148 Results from a study of streptozotocin-treated, Nrf2-knockout mice revealed reduced protection against inflammation, impaired kidney function, fibrosis, and oxidative damage.149
Focal Segmental Glomerulosclerosis
Although podocyte injury and loss may be the primary drivers of FSGS,150 inflammation is also thought to play a key role in disease progression.151,152 Involvement of inflammation in the etiology of FSGS was suggested by the finding of higher interstitial cluster of differentiation 3-positive T cells and macrophages in kidney biopsies from patients with FSGS.153 Oxidative stress also contributes to the pathogenesis of FSGS.154 Damage to podocytes leads to further injury mediated by cytokine release (e.g., TGF-β), which results in the recruitment of monocytes, macrophages, and T cells and enhanced expression and secretion of other cytokines (e.g., IL-1 and TNF) and chemokines.152 Baseline kidney function in patients with FSGS is inversely correlated with the extent of global sclerosis and tubulointerstitial fibrosis, including urinary excretion of IL-12, interferon-γ, IL-4, IL-5, and IL-13.155 The infiltration of inflammatory cells in FSGS results in the accumulation of mesangial ECM, which can cause glomerular collapse (“collapsing variant”).152 Damage to tubular epithelial cells leads to a transformation to mesenchymal cells, resulting in collagen matrix deposition and tubulointerstitial fibrosis.152,156 This proinflammatory and profibrotic milieu has been involved in the progression of FSGS and may cause glomerular scarring and eventual ESKD.157 A role for Nrf2 in the progression of FSGS was found in the Imai rat model, in which impaired Nrf2 signaling in conjunction with NF-κB activation promoted inflammation and oxidative stress, both of which were associated with progressive glomerulosclerosis.158
Conclusion
Mounting evidence suggests a significant role of inflammation and metabolic pathways in the progression of CKD of multiple etiologies.23 Pathway-crosstalk analysis of gene sets linked to estimated GFR reveals that most CKD signaling pathways aggregate in either an inflammation- or a metabolism-related cluster.23 Steady-state mRNA expression patterns across the spectrum of CKD are consistent with up-regulation of inflammatory genes.23 The Nrf2 pathway serves as a hub linking metabolic and inflammatory pathways in CKD.23 Repressed expression of this cytoprotective factor is linked to several pathogenic mechanisms known to promote fibrosis and progression of kidney disease, such as senescence, inflammation, mitochondrial dysfunction, and tissue hypoxia; therefore, Nrf2 activation is an attractive target for arresting progression of CKD.12,13,61,62 Augmenting the action of Nrf2 and its downstream mediators in CKD has the potential to attenuate, arrest, or even reverse the decline in kidney function.
Disclosure
PS: scientific advisory boards for Reata Pharmaceuticals, Inc., AstraZeneca, Vifor Pharma, and Baxter Healthcare. GMC: board of directors for Satellite Healthcare, Inc.; consultant/advisor for Akebia Therapeutics, Amgen, Ardelyx, Inc., AstraZeneca, Baxter Healthcare, CloudCath, Cricket Health, DiaMedica Therapeutics, Inc., Durect Corp, DxNow, Inc., Gilead Sciences, Inc., Miromatrix Medical, Inc., Outset Medical, Reata Pharmaceuticals, Inc., Sanifit, and Vertex Pharmaceuticals Inc. PD: medical advisory board for Reata Pharmaceuticals, Inc.; speakers bureau for BioPorto Inc.; key opinion leader for Alnylam Pharmaceuticals and Dicerna Pharmaceuticals; coinventor on submitted patents for the use of NGAL as a biomarker of kidney injury; licensing agreements with Abbott Diagnostics and BioPorto Inc. for the development of NGAL as a biomarker of kidney injury. AL: advisory committee for Reata Pharmaceuticals, Inc.; grants from Otsuka America Pharmaceutical, Inc., AstraZeneca, and Boehringer Ingelheim International GmbH. SPA: consultant advisor to Alexion Pharmaceuticals and Reata Pharmaceuticals, Inc. SB: advisory committee for Reata Pharmaceuticals, Inc. BAW: advisory committee for Reata Pharmaceuticals, Inc.; medical advisory committee of the Alport Syndrome Foundation; consultant for Bayer AG, Akebia Therapeutics, Relypsa, Inc., Amgen, FibroGen, Inc., and UpToDate, Inc.; research support from the National Institutes of Health and Baxter Healthcare.
Acknowledgments
This work received support from Reata Pharmaceuticals, Inc.
Author Contributions
PS, GMC, and BAW drafted the manuscript. PD, AL, SPA, and SP provided editorial insights.
Footnotes
Figure S1. Molecular mechanism of the Keap1-Nrf2 system during the oxidative stress response.76 Under normal conditions, Nrf2 is degraded and inactivated after being captured by Keap1 homodimers. Under conditions of oxidative stress, the interaction between Keap1 and Nrf2 is inactivated, resulting in decreased Nrf2 degradation. Nuclear translocation of stabilized Nrf2 allows for transcriptional activation of Nrf2 target genes. Cys, cysteine residues; Keap1, kelch-like ECH-associated protein 1; Nrf2, nuclear factor, erythroid 2 like 2; Ub, ubiquitin-proteasome–dependent degradation. Reproduced with permission from Nezu M et al. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45(6):473-483. Copyright © 2017 Karger Publishers, Basel, Switzerland.
Figure S2. Progression of kidney disease as part of premature aging processes in CKD.87 The toxic uremic milieu promotes inflammation and repressed expression of Nrf2, a phenomenon linked to oxidative stress, mitochondrial dysfunction, tissue hypoxia, and senescence. Evidence suggests that these features are major drivers of a prematurely aged phenotype in CKD, including early vascular aging (vascular calcification), sarcopenia, osteoporosis, heart failure, depression, and cognitive dysfunction. In addition, the same features may drive early aging in the kidney-by-kidney fibrosis and inflammation. In addition to hemodynamic and metabolic factors, inflammation and fibrosis may drive progression of kidney disease, creating a vicious circle. Nephrologists may intervene in this scenario by using established, novel, and putative future treatment strategies, such as ACEi/ARB, SGLT2i, Nrf2 agonist, and MR inhibitors. Angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker (ACEi/ARB); CKD, chronic kidney disease; MR, mineralocorticoid receptor; Nrf2, nuclear factor, erythroid 2 like 2; sodium-glucose transport protein 2 inhibitor (SGLT2i).
Supplementary Material
Figure S1. Molecular mechanism of the Keap1-Nrf2 system during the oxidative stress response76 Under normal conditions, Nrf2 is degraded and inactivated after being captured by Keap1 homodimers. Under conditions of oxidative stress, the interaction between Keap1 and Nrf2 is inactivated, resulting in decreased Nrf2 degradation. Nuclear translocation of stabilized Nrf2 allows for transcriptional activation of Nrf2 target genes. Cys, cysteine residues; Keap1, kelch-like ECH-associated protein-1; Nrf2, nuclear factor, erythroid 2 like 2; Ub, ubiquitin-proteasome–dependent degradation. Reproduced with permission from Nezu M et al. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45(6):473-483. Copyright © 2017 Karger Publishers, Basel, Switzerland.
Figure S2. Progression of kidney disease as part of premature aging processes in CKD.87 The toxic uremic milieu promotes inflammation and repressed expression of Nrf2, a phenomenon linked to oxidative stress, mitochondrial dysfunction, tissue hypoxia, and senescence. Evidence suggests that these features are major drivers of a prematurely aged phenotype in CKD, including early vascular aging (vascular calcification), sarcopenia, osteoporosis, heart failure, depression, and cognitive dysfunction. In addition, the same features may drive early aging in the kidney by kidney fibrosis and inflammation. In addition to hemodynamic and metabolic factors, inflammation and fibrosis may drive progression of kidney disease, creating a vicious circle. Nephrologists may intervene in this scenario by using established, novel, and putative future treatment strategies, such as ACEi/ARB, SGLT2i, Nrf2 agonist, and MR inhibitors. Angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker (ACEi/ARB); CKD, chronic kidney disease; MR, mineralocorticoid receptor; Nrf2, nuclear factor, erythroid 2 like 2; sodium-glucose transport protein 2 inhibitor (SGLT2i).
References
- 1.Helal I., Fick-Brosnahan G.M., Reed-Gitomer B., Schrier R.W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 2.Noone D., Licht C. An update on the pathomechanisms and future therapies of Alport syndrome. Pediatr Nephrol. 2013;28:1025–1036. doi: 10.1007/s00467-012-2272-z. [DOI] [PubMed] [Google Scholar]
- 3.Savige J. Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol. 2014;592:4013–4023. doi: 10.1113/jphysiol.2014.274449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright J.T., Jr., Bakris G., Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 5.SPRINT Research Group. Wright J.T., Jr., Williamson J.D. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert L.A., Kusek J.W., Greene T. Effects of blood pressure control on progressive renal disease in blacks and whites. Modification of Diet in Renal Disease Study Group. Hypertension. 1997;30:428–435. doi: 10.1161/01.hyp.30.3.428. [DOI] [PubMed] [Google Scholar]
- 7.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 8.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 9.Zheng C.M., Wang J.Y., Chen T.T. Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker monotherapy retard deterioration of renal function in Taiwanese chronic kidney disease population. Sci Rep. 2019;9:2694. doi: 10.1038/s41598-019-38991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson R.J., Rodriguez-Iturbe B. Rethinking progression of CKD as a process of punctuated equilibrium. Nat Rev Nephrol. 2018;14:411–412. doi: 10.1038/s41581-018-0016-4. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Ortega M., Rayego-Mateos S., Lamas S. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269–288. doi: 10.1038/s41581-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 12.Akchurin O.M., Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 13.Wei P.Z., Szeto C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta. 2019;496:108–116. doi: 10.1016/j.cca.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Imig J.D., Ryan M.J. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3:957–976. doi: 10.1002/cphy.c120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.J., Vaziri N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Ren Physiol. 2010;298:F662–F671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 16.Impellizzeri D., Esposito E., Attley J., Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD) Pharmacol Res. 2014;81:91–102. doi: 10.1016/j.phrs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10:493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 18.Ying T., Clayton P., Naresh C., Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrol. 2018;19:55. doi: 10.1186/s12882-018-0853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paisley K.E., Beaman M., Tooke J.E. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 2003;63:624–633. doi: 10.1046/j.1523-1755.2003.00768.x. [DOI] [PubMed] [Google Scholar]
- 20.Cravedi P., Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76:516–523. doi: 10.1111/bcp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagasu H., Sogawa Y., Kidokoro K. Bardoxolone methyl analog attenuates proteinuria-induced tubular damage by modulating mitochondrial function. FASEB J. 2019;33:12253–12263. doi: 10.1096/fj.201900217R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorriz J.L., Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando) 2012;26:3–13. doi: 10.1016/j.trre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Martini S., Nair V., Keller B.J. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol. 2014;25:2559–2572. doi: 10.1681/ASN.2013080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobo G., Lindholm B., Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl 3):iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himmelfarb J., Stenvinkel P., Ikizler T.A., Hakim R.M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 26.Kooman J.P., Dekker M.J., Usvyat L.A. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Ren Physiol. 2017;313:F938–F950. doi: 10.1152/ajprenal.00256.2017. [DOI] [PubMed] [Google Scholar]
- 27.Nlandu Khodo S., Dizin E., Sossauer G. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J Am Soc Nephrol. 2012;23:1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therrien F.J., Agharazii M., Lebel M., Larivière R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am J Nephrol. 2012;36:151–161. doi: 10.1159/000340033. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz S., Pergola P.E., Zager R.A., Vaziri N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grande M.T., Pérez-Barriocanal F., López-Novoa J.M. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm (Lond) 2010;7:19. doi: 10.1186/1476-9255-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Queisser N., Schupp N. Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic Biol Med. 2012;53:314–327. doi: 10.1016/j.freeradbiomed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Amdur R.L., Feldman H.I., Gupta J. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar A., Sun L., Klein B.E. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonelli M., Sacks F., Pfeffer M. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 36.Lv W., Booz G.W., Wang Y. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khwaja A., El Kossi M., Floege J., El Nahas M. The management of CKD: a look into the future. Kidney Int. 2007;72:1316–1323. doi: 10.1038/sj.ki.5002489. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 39.Eddy A.A. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 2014;4:2–8. doi: 10.1038/kisup.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 41.Mimura I., Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi J., Tanaka T., Nangaku M. Recent advances in understanding of chronic kidney disease. F1000Res. 2015;4 doi: 10.12688/f1000research.6970.1. F1000 Faculty Rev-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberg B.P., McMenamin E., Lucas F.L. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 44.Tecklenborg J., Clayton D., Siebert S., Coley S.M. The role of the immune system in kidney disease. Clin Exp Immunol. 2018;192:142–150. doi: 10.1111/cei.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullins L.J., Conway B.R., Menzies R.I. Renal disease pathophysiology and treatment: contributions from the rat. Dis Model Mech. 2016;9:1419–1433. doi: 10.1242/dmm.027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlondorff D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008;74:860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 47.Falke L.L., Gholizadeh S., Goldschmeding R. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat Rev Nephrol. 2015;11:233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 48.Lee S.A., Noel S., Sadasivam M. Role of immune cells in acute kidney injury and repair. Nephron. 2017;137:282–286. doi: 10.1159/000477181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwok S.K., Tsokos G.C. New insights into the role of renal resident cells in the pathogenesis of lupus nephritis. Korean J Intern Med. 2018;33:284–289. doi: 10.3904/kjim.2017.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitching A.R., Hutton H.L. The players: cells involved in glomerular disease. Clin J Am Soc Nephrol. 2016;11:1664–1674. doi: 10.2215/CJN.13791215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jourde-Chiche N., Fakhouri F., Dou L. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15:87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Guerrero C., Hernández-Vargas P., López-Franco O. Mesangial cells and glomerular inflammation: from the pathogenesis to novel therapeutic approaches. Curr Drug Targets Inflamm Allergy. 2005;4:341–351. doi: 10.2174/1568010054022169. [DOI] [PubMed] [Google Scholar]
- 53.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 54.Warady B.A., Agarwal R., Bangalore S. Alport syndrome classification and management. Kidney Med. 2020;2:639–649. doi: 10.1016/j.xkme.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamawaki K., Kanda H., Shimazaki R. Nrf2 activator for the treatment of kidney diseases. Toxicol Appl Pharmacol. 2018;360:30–37. doi: 10.1016/j.taap.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 56.Aminzadeh M.A., Reisman S.A., Vaziri N.D. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44:570–578. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 58.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sayers R., Kalluri R., Rodgers K.D. Role for transforming growth factor-beta1 in Alport renal disease progression. Kidney Int. 1999;56:1662–1673. doi: 10.1046/j.1523-1755.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.B., Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;(119):S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiley C.D. Role of senescent renal cells in pathophysiology of diabetic kidney disease. Curr Diab Rep. 2020;20:33. doi: 10.1007/s11892-020-01314-y. [DOI] [PubMed] [Google Scholar]
- 62.Fine L.G., Norman J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 63.Liu M., Reddy N.M., Higbee E.M. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85:134–141. doi: 10.1038/ki.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnaper H.W., Hayashida T., Poncelet A.C. It’s a Smad world: regulation of TGF-beta signaling in the kidney. J Am Soc Nephrol. 2002;13:1126–1128. doi: 10.1681/ASN.V1341126. [DOI] [PubMed] [Google Scholar]
- 65.Witasp A., Ekström T.J., Lindholm B. Novel insights from genetic and epigenetic studies in understanding the complex uraemic phenotype. Nephrol Dial Transplant. 2014;29:964–971. doi: 10.1093/ndt/gft428. [DOI] [PubMed] [Google Scholar]
- 66.Luttropp K., Debowska M., Lukaszuk T. Genotypic and phenotypic predictors of inflammation in patients with chronic kidney disease. Nephrol Dial Transplant. 2016;31:2033–2040. doi: 10.1093/ndt/gfw066. [DOI] [PubMed] [Google Scholar]
- 67.Choi B.H., Kang K.S., Kwak M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules. 2014;19:12727–12759. doi: 10.3390/molecules190812727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stenvinkel P., Meyer C.J., Block G.A. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2–related factor 2—lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transplant. 2020;35:2036–2045. doi: 10.1093/ndt/gfz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang T., Huang Z., Lin Y. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuadrado A., Manda G., Hassan A. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 71.Ebert T., Pawelzik S., Witasp A. Inflammation and premature ageing in chronic kidney disease. Toxins (Basel) 2020;12:227. doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nezu M., Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int J Mol Sci. 2020;21:2951. doi: 10.3390/ijms21082951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guerrero-Hue M., Rayego-Mateos S., Vazquez-Carballo C. Protective role of Nrf2 in renal disease. Antioxidants (Basel) 2020;10:39. doi: 10.3390/antiox10010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pedruzzi L.M., Stockler-Pinto M.B., Leite M., Jr., Mafra D. Nrf2-keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie. 2012;94:2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 76.Nezu M., Suzuki N., Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45:473–483. doi: 10.1159/000475890. [DOI] [PubMed] [Google Scholar]
- 77.Kobayashi E.H., Suzuki T., Funayama R. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed S.M., Luo L., Namani A. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Kong X., Thimmulappa R., Craciun F. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184:928–938. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W., Khor T.O., Xu C. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volonte D., Liu Z., Musille P.M. Inhibition of nuclear factor-erythroid 2–related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell. 2013;24:1852–1862. doi: 10.1091/mbc.E12-09-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duan W., Zhang R., Guo Y. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim. 2009;45:388–397. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 83.Luo X., Zhou J., Wang Z. An inhibitor role of Nrf2 in the regulation of myocardial senescence and dysfunction after myocardial infarction. Life Sci. 2020;259:118199. doi: 10.1016/j.lfs.2020.118199. [DOI] [PubMed] [Google Scholar]
- 84.Kubben N., Zhang W., Wang L. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki T., Uruno A., Yumoto A. Nrf2 contributes to the weight gain of mice during space travel. Commun Biol. 2020;3:496. doi: 10.1038/s42003-020-01227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong D., Cai G.Y., Ning Y.C. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget. 2017;8:16109–16121. doi: 10.18632/oncotarget.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kooman J.P., Kotanko P., Schols A.M. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10:732–742. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 88.Al-Sawaf O., Clarner T., Fragoulis A. Nrf2 in health and disease: current and future clinical implications. Clin Sci (Lond) 2015;129:989–999. doi: 10.1042/CS20150436. [DOI] [PubMed] [Google Scholar]
- 89.Lu M.C., Zhao J., Liu Y.T. CPUY192018, a potent inhibitor of the Keap1-Nrf2 protein–protein interaction, alleviates renal inflammation in mice by restricting oxidative stress and NF-κB activation. Redox Biol. 2019;26:101266. doi: 10.1016/j.redox.2019.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng Y.L., Chen H., Chen D.Q. Activated NF-κB/Nrf2 and Wnt/β-catenin pathways are associated with lipid metabolism in CKD patients with microalbuminuria and macroalbuminuria. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2317–2332. doi: 10.1016/j.bbadis.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 91.Lu Y., Sun Y., Liu Z. Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Sci Transl Med. 2020;12:eaba3613. doi: 10.1126/scitranslmed.aba3613. [DOI] [PubMed] [Google Scholar]
- 92.Juul-Nielsen C., Shen J., Stenvinkel P., Scholze A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: alterations, interventions, and relation to morbidity [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab031 [DOI] [PubMed]
- 93.Leal V.O., Saldanha J.F., Stockler-Pinto M.B. NRF2 and NF-κB mRNA expression in chronic kidney disease: a focus on nondialysis patients. Int Urol Nephrol. 2015;47:1985–1991. doi: 10.1007/s11255-015-1135-5. [DOI] [PubMed] [Google Scholar]
- 94.Tsai P.Y., Ka S.M., Chao T.K. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic Biol Med. 2011;50:1503–1516. doi: 10.1016/j.freeradbiomed.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 95.Aminzadeh M.A., Reisman S.A., Vaziri N.D. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol. 2013;1:527–531. doi: 10.1016/j.redox.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rinaldi Tosi M.E., Bocanegra V., Manucha W. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chaperones. 2011;16:57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Palmer S.C., Tendal B., Mustafa R.A. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hesp A.C., Schaub J.A., Prasad P.V. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020;98:579–589. doi: 10.1016/j.kint.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elkazzaz S.K., Khodeer D.M., El Fayoumi H.M., Moustafa Y.M. Role of sodium glucose cotransporter type 2 inhibitors dapagliflozin on diabetic nephropathy in rats; inflammation, angiogenesis and apoptosis [e-pub ahead of print]. Life Sci. https://doi.org/10.1016/j.lfs.2021.119018 [DOI] [PubMed]
- 100.Yaribeygi H., Atkin S.L., Butler A.E., Sahebkar A. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234:3231–3237. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 101.Arab H.H., Al-Shorbagy M.Y., Saad M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. 2021;335:109368. doi: 10.1016/j.cbi.2021.109368. [DOI] [PubMed] [Google Scholar]
- 102.Forbes J.M., Thorburn D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14:291–312. doi: 10.1038/nrneph.2018.9. [DOI] [PubMed] [Google Scholar]
- 103.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breda C.N.S., Davanzo G.G., Basso P.J. Mitochondria as central hub of the immune system. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banoth B., Cassel S.L. Mitochondria in innate immune signaling. Transl Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jung J., Zeng H., Horng T. Metabolism as a guiding force for immunity. Nat Cell Biol. 2019;21:85–93. doi: 10.1038/s41556-018-0217-x. [DOI] [PubMed] [Google Scholar]
- 107.West A.P., Brodsky I.E., Rahner C. TLR signaling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galvan D.L., Green N.H., Danesh F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kasai S., Shimizu S., Tatara Y. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmström K.M., Baird L., Zhang Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Daenen K., Andries A., Mekahli D. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34:975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- 115.Tin A., Grams M.E., Ashar F.N. Association between mitochondrial DNA copy number in peripheral blood and incident CKD in the atherosclerosis risk in communities study. J Am Soc Nephrol. 2016;27:2467–2473. doi: 10.1681/ASN.2015060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu C., Gidlund E.K., Witasp A. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am J Physiol Ren Physiol. 2019;317:F1122–F1131. doi: 10.1152/ajprenal.00202.2019. [DOI] [PubMed] [Google Scholar]
- 117.Yoon Y.M., Go G., Yun C.W. Melatonin suppresses renal cortical fibrosis by inhibiting cytoskeleton reorganization and mitochondrial dysfunction through regulation of miR-4516. Int J Mol Sci. 2020;21:5323. doi: 10.3390/ijms21155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Console L., Scalise M., Giangregorio N. The link between the mitochondrial fatty acid oxidation derangement and kidney injury. Front Physiol. 2020;11:794. doi: 10.3389/fphys.2020.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cosgrove D., Meehan D.T., Grunkemeyer J.A. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- 120.Cosgrove D. Glomerular pathology in Alport syndrome: a molecular perspective. Pediatr Nephrol. 2012;27:885–890. doi: 10.1007/s00467-011-1868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cosgrove D., Rodgers K., Meehan D. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chertow G.M., Appel G.B., Andreoli S. Study design and baseline characteristics of the CARDINAL trial: a phase 3 study of Bardoxolone methyl in patients with Alport syndrome. Am J Nephrol. 2021;52:180–189. doi: 10.1159/000513777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li X., editor. Polycystic Kidney Disease. Codon Publications; Kansas City, KS: 2015. [PubMed] [Google Scholar]
- 124.Phillips J.K., Hopwood D., Loxley R.A. Temporal relationship between renal cyst development, hypertension and cardiac hypertrophy in a new rat model of autosomal recessive polycystic kidney disease. Kidney Blood Press Res. 2007;30:129–144. doi: 10.1159/000101828. [DOI] [PubMed] [Google Scholar]
- 125.Chen L., Zhou X., Fan L.X. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest. 2015;125:2399–2412. doi: 10.1172/JCI80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karihaloo A., Koraishy F., Huen S.C. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2011;22:1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park E.Y., Seo M.J., Park J.H. Effects of specific genes activating RAGE on polycystic kidney disease. Am J Nephrol. 2010;32:169–178. doi: 10.1159/000315859. [DOI] [PubMed] [Google Scholar]
- 128.Gardner K.D., Jr., Burnside J.S., Elzinga L.W., Locksley R.M. Cytokines in fluids from polycystic kidneys. Kidney Int. 1991;39:718–724. doi: 10.1038/ki.1991.87. [DOI] [PubMed] [Google Scholar]
- 129.Li X., Magenheimer B.S., Xia S. A tumor necrosis factor-alpha–mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med. 2008;14:863–868. doi: 10.1038/nm1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 131.Wu M.Y., Chen C.S., Yiang G.T. The emerging role of pathogenesis of IgA nephropathy. J Clin Med. 2018;7:225. doi: 10.3390/jcm7080225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yeo S.C., Cheung C.K., Barratt J. New insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol. 2018;33:763–777. doi: 10.1007/s00467-017-3699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodrigues J.C., Haas M., Reich H.N. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12:677–686. doi: 10.2215/CJN.07420716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan L.Y., Leung J.C., Tsang A.W. Activation of tubular epithelial cells by mesangial-derived TNF-alpha: glomerulotubular communication in IgA nephropathy. Kidney Int. 2005;67:602–612. doi: 10.1111/j.1523-1755.2005.67116.x. [DOI] [PubMed] [Google Scholar]
- 135.Lai K.N., Leung J.C., Chan L.Y. Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol Dial Transplant. 2009;24:62–72. doi: 10.1093/ndt/gfn441. [DOI] [PubMed] [Google Scholar]
- 136.Hara M., Yanagihara T., Kihara I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein–Henoch purpura nephritis. Clin J Am Soc Nephrol. 2007;2:231–238. doi: 10.2215/CJN.01470506. [DOI] [PubMed] [Google Scholar]
- 137.Wang C., Ye Z., Peng H. Effect of aggregated immunoglobulin A1 from immunoglobulin A nephropathy patients on nephrin expression in podocytes. Nephrology (Carlton) 2009;14:213–218. doi: 10.1111/j.1440-1797.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 138.Lai K.N., Leung J.C., Chan L.Y. Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Ren Physiol. 2008;294:F945–F955. doi: 10.1152/ajprenal.00423.2007. [DOI] [PubMed] [Google Scholar]
- 139.Yang S.M., Ka S.M., Hua K.F. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic Biol Med. 2013;61:285–297. doi: 10.1016/j.freeradbiomed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 140.Alicic R.Z., Johnson E.J., Tuttle K.R. Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25:181–191. doi: 10.1053/j.ackd.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 141.García-García P.M., Getino-Melián M.A., Domínguez-Pimentel V., Navarro-González J.F. Inflammation in diabetic kidney disease. World J Diabetes. 2014;5:431–443. doi: 10.4239/wjd.v5.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Navarro-González J.F., Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 143.Navarro-González J.F., Mora-Fernández C., Muros de Fuentes M., García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 144.Wolkow P.P., Niewczas M.A., Perkins B. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19:789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Donate-Correa J., Martín-Núñez E., Muros-de-Fuentes M. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. doi: 10.1155/2015/948417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Niewczas M.A., Pavkov M.E., Skupien J. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805–813. doi: 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dalla Vestra M., Mussap M., Gallina P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(suppl 1):S78–S82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- 148.Arellano-Buendía A.S., Tostado-González M., García-Arroyo F.E. Anti-inflammatory therapy modulates Nrf2-Keap1 in kidney from rats with diabetes. Oxid Med Cell Longev. 2016;2016:4693801. doi: 10.1155/2016/4693801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong L., Wang Y., Luo M. Prevention of streptozotocin-induced diabetic nephropathy by MG132: possible roles of Nrf2 and IκB. Oxid Med Cell Longev. 2017;2017:3671751. doi: 10.1155/2017/3671751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiggins R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 151.Abbate M., Zoja C., Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 152.Reidy K., Kaskel F.J. Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22:350–354. doi: 10.1007/s00467-006-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benz K., Büttner M., Dittrich K. Characterisation of renal immune cell infiltrates in children with nephrotic syndrome. Pediatr Nephrol. 2010;25:1291–1298. doi: 10.1007/s00467-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 154.Musante L., Candiano G., Petretto A. Active focal segmental glomerulosclerosis is associated with massive oxidation of plasma albumin. J Am Soc Nephrol. 2007;18:799–810. doi: 10.1681/ASN.2006090965. [DOI] [PubMed] [Google Scholar]
- 155.Stangou M., Spartalis M., Daikidou D.V. Impact of Th1 and Th2 cytokines in the progression of idiopathic nephrotic syndrome due to focal segmental glomerulosclerosis and minimal change disease. J Nephropathol. 2017;6:187–195. doi: 10.15171/jnp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harris R.C., Neilson E.G. Toward a unified theory of renal progression. Annu Rev Med. 2006;57:365–380. doi: 10.1146/annurev.med.57.121304.131342. [DOI] [PubMed] [Google Scholar]
- 157.Kronbichler A., Leierer J., Oh J. Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. BioMed Res Int. 2016;2016:2150451. doi: 10.1155/2016/2150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim H.J., Sato T., Rodríguez-Iturbe B., Vaziri N.D. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2–related factor 2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther. 2011;337:583–590. doi: 10.1124/jpet.110.175828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.