Abstract
Backgrounds
As one of the most important cereals, wheat (Triticum aestivum) can cause severe allergic reactions, such as baker's asthma, allergic rhinitis, and atopic dermatitis. A growing number of people are developing allergies to Chinese wheat; however, only a few wheat cultivars have been screened on allergenicity in China.
Objective
The aim of the present study was to assess the allergenicity of different Chinese wheat cultivars and characterize wheat allergen profiles of patients with allergic rhinitis.
Methods
We determined protein (soluble protein, gliadin, and glutenin) composition in Chinese wheat by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the immunoglobulin E (IgE) binding capacity by enzyme-linked immunosorbent assay (ELISA) and Western blot using 10 positive sera from wheat allergy patients. We identified 5 gel bands with significant IgE binding capacity using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results
Soluble protein, albumin, and globulin, showed the highest allergenicity, followed by gliadin, while glutenin only had slight allergenicity. In soluble protein, 5 protein bands with molecular weights of 27, 28, 53, 58, and 62 kDa showed very significant allergenicity. Meanwhile, the relative abundances of 28 kDa-protein and 58 kDa-protein were significantly positively correlated with the IgE-binding capacity of Chinese wheat cultivars, which were identified as rRNA N-glycosidase and β-amylase, respectively, among other proteins in those highly complex gel bands.
Conclusion and clinical relevance
28 kDa-protein (rRNA N-glycosidase) and 58 kDa-protein (β-amylase) were speculated to be the main allergens of Chinese wheat causing baker's asthma and allergic rhinitis. These results provide new insights into the prevention and treatment of wheat allergy and the development of hypoallergenic wheat products, whose clinical significance is worth further evaluation.
Keywords: Chinese wheat, Main allergens, Soluble protein, Allergic rhinitis
Introduction
Triticum aestivum (bread wheat) is the most widely planted crop in the world, providing a major contribution to global food and nutrition security.1 The wheat is rich in starch, protein, minerals, B vitamins, and the biologically active ingredient which has high nutritional value and high palatability. Unfortunately, wheat is commonly identified as one of the “big eight” categories of food allergens, which can initiate both immunoglobulin E (IgE) and non-IgE mediated food hypersensitivity in allergic individuals. Diseases caused by wheat allergies affect approximately 0.4% of individuals worldwide and up to 3.6% of Europeans.2 According to epidemiological studies, wheat allergy affects 0.4% of American adults,3 up to 3% of the general American pediatric population2 and as many as 3.6% children in northern China.4
The protein content of wheat equals 10–15%, and wheat protein can be classified into 4 classes: water-soluble albumin, salt-soluble globulin, ethanol-soluble gliadin and urea, detergent, or potassium hydroxide (KOH)-soluble glutenin,5 in which albumin and globulin are called soluble protein. To date, 28 proteins have been registered as allergens by the World Health Organization and International Union of Immunological Societies (WHO/IUIS, www.foodallergy.org). In general, these allergens cover all 4 classes of wheat proteins. Depending on the different routes of allergen exposure, ingestion of wheat can induce various IgE-mediated adverse reactions, including the following clinical symptoms: baker's asthma, wheat-dependent exercise-induced anaphylaxis (WDEIA), allergic rhinitis, and atopic dermatitis.6,7
Recently, wheat allergens and related diseases have been extensively studied, but no definitive conclusion has been reached regarding the relationship between wheat allergens and clinical symptoms.7,8 In Japan, Matsuo reported that γ-gliadin and ω-5-gliadin were the major allergens that trigger WDEIA.9 Maruyama found that glutenin had the highest allergenicity, followed by gliadins.10 It reveals that different wheat allergic patients have different sensitivities to different proteins. In France, Battais found that globulin and albumin were the major allergens inducing atopic dermatitis in children, and ω-5-gliadin was the major allergen inducing WDEIA and urticaria in adults.11 In Australia, Le reported that Australians showed a low probability of WDEIA, although ω-5-gliadin was the main allergen inducing WDEIA.12 Hence, the different allergen-associated clinical symptoms from different wheat species or patients in different regions are not completely identical.
According to the statistics, a growing number of people have developed allergies to Chinese wheat in recent decades.13 However, these investigators gathered data from western countries, and only a few Chinese wheat cultivars have been screened on allergenicity. In China, there are thousands of wheat cultivars grown, with 18 major cultivar groups being the most popular in different regions. Wheat flour has been widely used in processed foods such as noodles, bread, biscuits, and so on. Therefore, there is an urgent need to analyze the major allergens of Chinese wheat to enforce wheat allergen management.
In the present study, we aim to assess the allergenicity of 18 major Chinese wheat cultivars and characterize wheat allergen profiles of patients with allergic rhinitis. The major allergens of Chinese wheat were further explored, which can provoke allergic rhinitis in sensitized individuals. The present investigation is an effort towards the assessment of allergenicity and allergen profiles of Chinese wheat, which would be an important contribution in the direction of developing hypoallergenic wheat products and a therapeutic approach for wheat allergy.
Materials and methods
Materials
Raw Chinese wheat seeds were obtained from Henan Academy of Agricultural Sciences, China. The 18 Chinese wheat cultivars were Zhong 1211, Zhou 32, Zheng 366, Huai 40, Luo 29, Yan 4110, Bai 64, Feng 20, Bai 4199, Zhou 18, Luo 26, Zhou 27, Zhou 36, Bai 58, Zheng 113, Zheng 136, Bai 207, and Xu 918.
Patients and sera
Human sera from volunteer patients with wheat allergy were taken from PlasmaLab (USA) and listed in Table S1. The 10 patients, 4 females and 6 males, were aged 16 to 50 and the average age was 30 years old. The sIgE values of all sera were determined by ImunoCAP 100 (Phadia, Sweden). All patients had sIgE to wheat >0.5 kU/l. Most of them were in class 3 allergy (>3.5 kU/l), and 2 patients were in class 4 allergy (>17.5 kU/l). All patients had allergic rhinitis. Human serum samples from healthy subjects (negative in sIgE with wheat flour) were obtained from PlasmaLab (USA).
Wheat protein extraction
Soluble protein: As previously reported,14 wheat flour was degreased by acetone at a 1:10 (w/v) ratio at 4 °C until the solution was clear and transparent. Then, wheat flour (4 g) was suspended in 24 mL of phosphate-buffered saline (PBS) buffer (10 mM, pH 7.4) and left standing for 24 h at 4 °C. The supernatant was obtained after centrifugation at 12,000 g for 15 min at 4 °C (Beckman Coulter, Mississauga, ON).
Gliadin: As previously reported,15 4 g wheat flour was suspended in 28 mL of sodium chloride (0.5 mol/L) and ultrasonically oscillated for 30 min, and the precipitate was obtained after centrifugation at 12,000 g for 15 min at 4 °C in triplicate. The precipitate was then ultrasonically oscillated in ultrapure water at a 1:7 (w/v) ratio for 30 min, obtained after centrifugation at 12,000 g for 15 min at 4 °C in triplicate. Then, the precipitate was mixed with 70% ethyl alcohol at a 1:2 (w/v) ratio and ultrasonically oscillated for 30 min. The extract was centrifuged at 12,000 g for 15 min at 4 °C, and the supernatant was obtained.
Glutenin: As previously reported,16 wheat flour was ultrasonically oscillated in N-propanol at a 1:5 (w/v) ratio for 30 min to remove gliadin. The mixture was centrifuged at 12,000 g for 15 min at 4 °C and the precipitate was obtained. The precipitate was then mixed with 5 mL of extractant A (50% N-propanol, 1% DTT) at 60 °C for 30 min, and the supernatant was obtained after centrifugation at 12,000 g for 15 min at 4 °C. After that, 1.25 mL of extractant B (100% N-propanol, 1% DTT) was added to the supernatant, and the precipitate was collected after centrifugation at 12,000 g for 15 min at 4 °C. Meanwhile, the supernatant was mixed with 6.25 mL of extractant B and precipitate was collected after centrifugation at 12,000 g for 15 min at 4 °C. Then, both precipitates were dissolved in 0.5 mol/L of acetic acid.
The concentrations of soluble protein, gliadin, and glutenin were determined by bicinchoninic acid (BCA) assay (Keygen Biotech, Jiangsu, China), and the components of wheat extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using AlphaView SA 3.4.0 software (Proteinsimple, California, USA). The SDS-PAGE was conducted using 5% stacking gel and 12% separating gel and stained with Coomassie blue.
Enzyme-linked immunosorbent assay (ELISA) analysis
Wheat protein extract (15 μg/mL) was immobilized in 96-well microtiter plates overnight at 4 °C. After incubating with 200 μL of 5% bovine serum albumin (BSA) for 2 h at 25 °C, the plate was incubated with 100 μL of pooled sera from 10 patients with a normal serum as a negative control at dilution of 1:30 (v/v) for 1.5 h at 37 °C. The sIgE values of normal serum to wheat, codfish, and shrimp were <0.35 kU/l, 31.8 kU/l and 26 kU/l, respectively. After that, 100 μL of horseradish peroxidase (HRP)-conjugated goat anti-human IgE (1:5,000, v/v) was added and incubated for 1.5 h at 37 °C. Then, 100 μL of tetramethylbenzidine (TMB) solution was added and the plates were incubated for 15 min at 37 °C. Color development reaction was stopped by 50 μL of 2 M H2SO4 solution. The optical density of each well was measured at 450 nm with a microplate reader (Spectra Max i3x; Molecular Devices, USA).
Western blot analysis
Wheat protein extract (1 mg/mL) was run in a polyacrylamide gel (5% and 12%) under denaturing and reducing conditions in duplicate gels. After that, the gel was transferred to a polyvinylidene fluoride (PVDF) membrane (Amersham Hybond P, 0.45 μm, GE, USA) and blocked with 5% BSA at room temperature for 1 h. After washing 3 times with 200 μL of TBST (0.1% Tween-20 in TBS), the membrane was incubated with 1:10 (v/v) dilution of positive sera for 1.5 h at 37 °C. After washing 3 times with TBST, a dilution of 1:5000 (v/v) of HRP-conjugated goat anti-human IgE was added and incubated for 1 h at 37 °C. Then, the membrane was washed 3 times with TBST and developed using electrochemiluminescence (ECL) luminous liquid and for 1–2 min. The membrane was then scanned using AlphaView SA 3.4.0 software.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
The bands of the 27, 28, 53, 58, and 62 kDa-proteins were excised, destained, and digested with trypsin for LC-MS/MS analysis performed using a liquid chromatograph (Easy-nLC 1000, Thermo Fisher, USA) and a mass spectrometer (LTQ Obitrap ETD, Thermo Fisher, USA). The LC-MS/MS analysis was performed by Micrometer Biotech Company (Hangzhou, China). The tryptic peptides were dissolved in solvent A (0.1% formic acid), and loaded onto a home-made C18 reversed-phase analytical column (1.8 mm, 0.15 × 1,00 mm). The elution gradient was comprised of an increase from 4% to 25% solvent B (100% acetonitrile) over 80 min. Then, 25%–35% solvent B was eluted for 4 min, climbed to 90% for 2 min, and held at 90% for the last 8 min. All procedures were performed at a flow rate of 300 nL/min. The mass spectrometry parameters were set as follows: spray voltage, 2.20 KV; capillary temperature, 320 °C, and normalized collision energy, 50%, and the search parameters were set as follows: database, UniProt-wheat-Triticum aestivum, enzyme, trypsin; allowance, up to 2 missed cleavage; precursor and fragment errors, 0.02 Da; false discovery rate, 0.01; mass range, 350 to 1800 for full scan; fragmentation type, HCD. A data-dependent procedure that alternated between one MS scan followed by 20 MS/MS scans with 15.0 s dynamic exclusion. The resulting MS/MS data were processed using MaxQuant with an integrated Andromeda search engine (v.1.5.2.8). Proteomic mass spectrometry data are available in PRIDE repository under dataset identiier PXD025828.
Epitope's prediction
The amino acid sequence of rRNA N-glycosidase (Accession: A0A3B6LWE2) and β-amylase (Accession: A0A3B6IYD4) from Triticum aestivum was obtained from NCBI (https://www.ncbi.nlm.nih.gov/). Three online servers, IEDB (http://www.iedb.org/), ABCpred (https://webs.iiitd.edu.in/raghava/abcpred/) and IMED (http://imed.med.ucm.es/Tools/antigenic.pl), were used to predict peptide segment of B cell epitopes.
Statistical analysis
All the quantitative results were analyzed with 3 replicates according to a completely randomized design. Statistical significance of the data was determined by one-way analysis of variance using SPSS software (17.0, SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant. The Pearson correlation coefficient was calculated by SPSS software (17.0, SPSS Inc., Chicago, IL, USA).
Results
Characterization of proteins in Chinese wheat
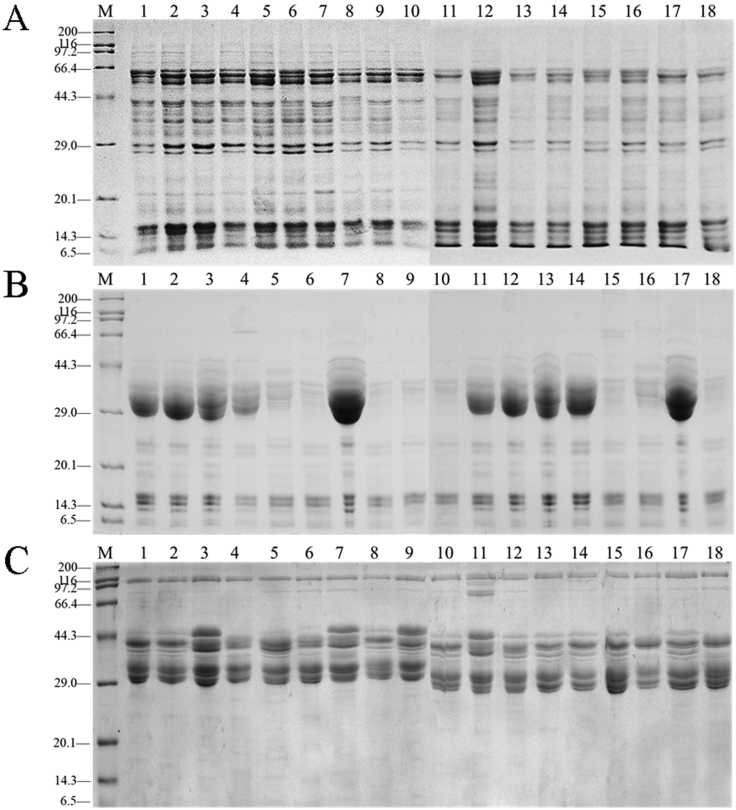
The average contents of extracted soluble protein, gliadin, and glutenin were 20.68, 20.46, and 37.87 mg/g dry wheat in 18 Chinese wheat cultivars, respectively. To determine the components of Chinese wheat protein, we separated the crude extracts of soluble protein, gliadin, and glutenin from 18 main Chinese wheat cultivars by SDS-PAGE. As shown in Fig. 1, almost all of the 18 Chinese wheat cultivars contained 25 protein bands with molecular weights ranging from approximately 9 to 105 kDa in soluble protein. The protein composition and relative content of soluble proteins can be shown in Fig. S1. Although the absolute content of proteins may not be accurate calculated by SDS-PAGE, the relative content was reliable. In addition, the ω-5-gliadin (55–66.3 kDa), ω-1,2-gliadin (39–42 kDa), γ-gliadin (36–45 kDa), αβ-gliadin (25–45 kDa), and such low molecular weight gliadin were presented in the crude extract of gliadin, which was consistent with the previous description.17 Moreover, we can visually observe 4 protein bands of high-molecular-weight glutenin subunits HMW-GS with molecular weights between 90 and 120 kDa and 3 protein bands of low-molecular-weight glutenin subunits LWM-GS with molecular weights between 40 and 70 kDa in the crude extract of glutenin.16 No significant differences were found in protein composition and relative abundances of soluble protein and glutenin between different Chinese wheat cultivars, while the relative abundance of gliadin was significantly different, especially proteins with molecular weights between 29 and 44.3 kDa.
Fig. 1.
SDS-PAGE of soluble protein (A), gliadin (B) and glutenin (C) in 18 major Chinese wheat cultivars. (M, Marker; lane 1, Zhong 1211; lane 2, Zhou 32; lane 3, Zheng 366; lane 4, Huai 40; lane 5, Luo 29; lane 6, Yan 4110; lane 7, Bai 64; lane 8, Feng 20; lane 9, Bai 4199; lane 10, Zhou 18; lane 11, Luo 26; lane 12, Zhou 27; lane 13, Zhou 36; lane 14, Bai 58; lane 15, Zheng 113; lane 16, Zheng 136; lane 17, Bai 207; lane 18, Xu 918.)
Allergenicity assessment of Chinese wheat
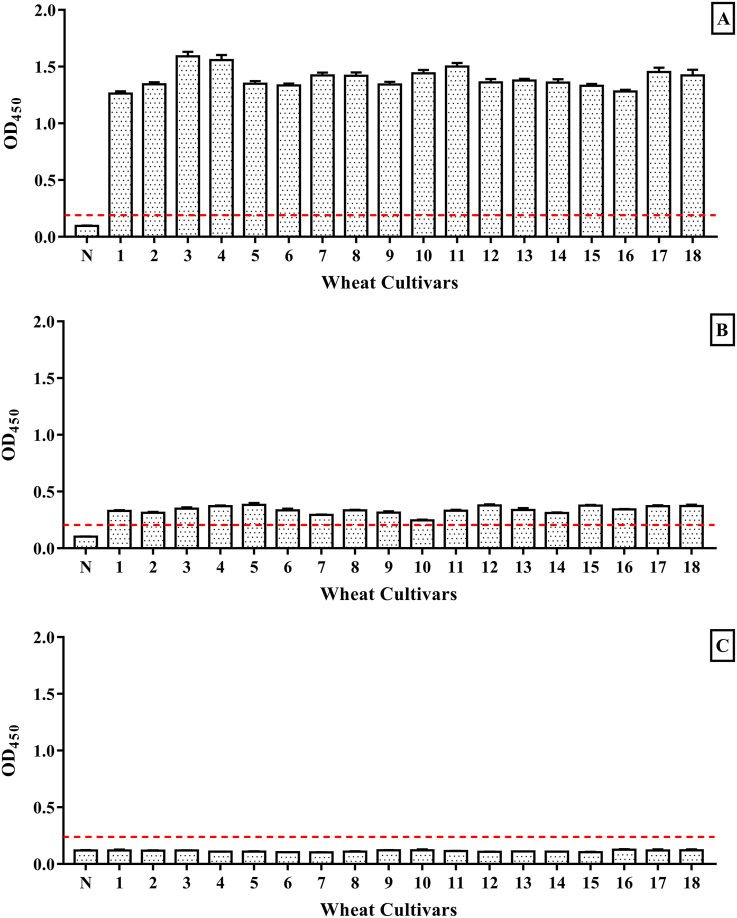
The IgE binding capacity of 3 classes of proteins in each Chinese wheat cultivar was defined by the absorbance (optical density (OD)450) in the indirect ELISA using a sera pool of 10 patients. As shown in Fig. 2A, all soluble proteins of 18 Chinese wheat cultivars demonstrated significantly high IgE binding capacity, with the OD values ranging from 1.263 to 1.591, indicating similar IgE binding capacity between different Chinese wheat cultivars. Among them, 3 Chinese wheat cultivars represented higher values, the Zheng 366 (No. 3) type had the highest IgE binding capacity, followed by Huai 40 (No. 4) and Luo 26 (No. 11). On the contrary, as Fig. 2B shown, all gliadins from 18 Chinese wheat cultivars had weak IgE binding capacities, with relatively low OD value ranging from 0.1633 to 0.2553. Similarly, no patients were significantly allergic to glutenin in each Chinese wheat cultivar (Fig. 2C), showing the lowest IgE binding capacity. Consequently, among the 3 classes of proteins in Chinese wheat, soluble protein showed the highest IgE binding capacity and potential allergenicity, while the gliadin and glutenin only exhibited low capacity and potential allergenicity.
Fig. 2.
The IgE binding capacity of soluble protein (A), gliadin (B), and glutenin (C) in 18 Chinese wheat cultivars. (Note: N, negative control; 1, Zhong 1211; 2, Zhou 32; 3, Zheng 366; 4, Huai 40; 5, Luo 29; 6, Yan 4110; 7, Bai 64; 8, Feng 20; 9, Bai 4199; 10, Zhou 18; 11, Luo 26; 12, Zhou 27; 13, Zhou 36; 14, Bai 58; 15, Zheng 113; 16, Zheng 136; 17, Bai 207; 18, Xu 918. In Fig.s 2A and 2B, all experimental groups were extremely significant differences compared to negative control. P < 0.0001.) The results were represented as mean ± SD from 3 replicates. The dashed line indicates the limit for positive values
Allergen composition analysis in Chinese wheat
To investigate the allergen composition of 18 Chinese wheat cultivars in patients with allergic rhinitis, the allergenicity of potential allergens in soluble protein was measured by Western blot, the correlation analysis between the protein contents, and IgE binding capacity.
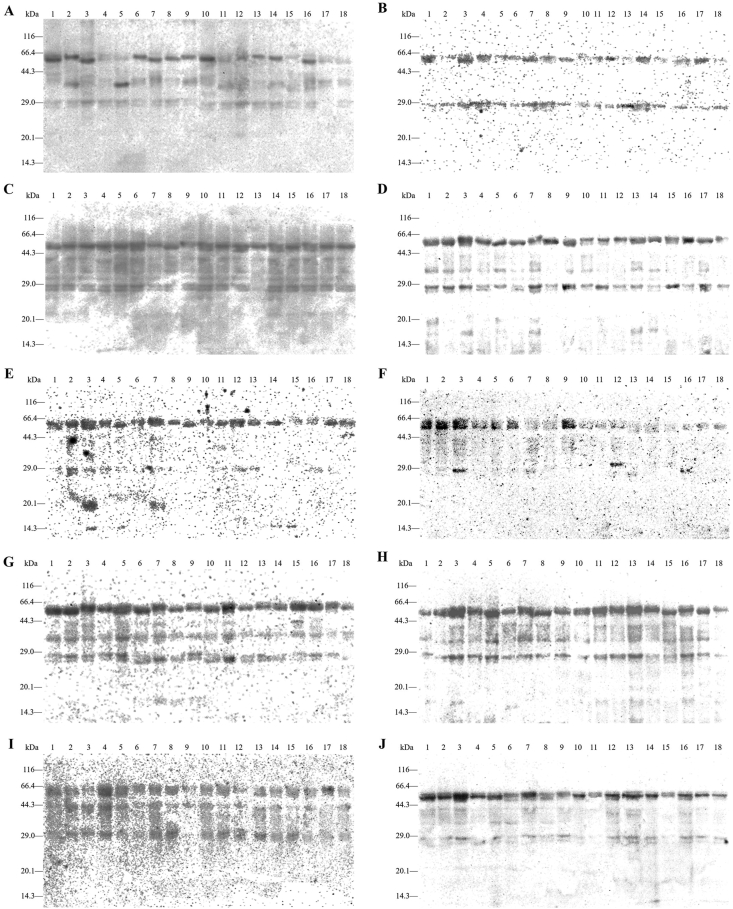
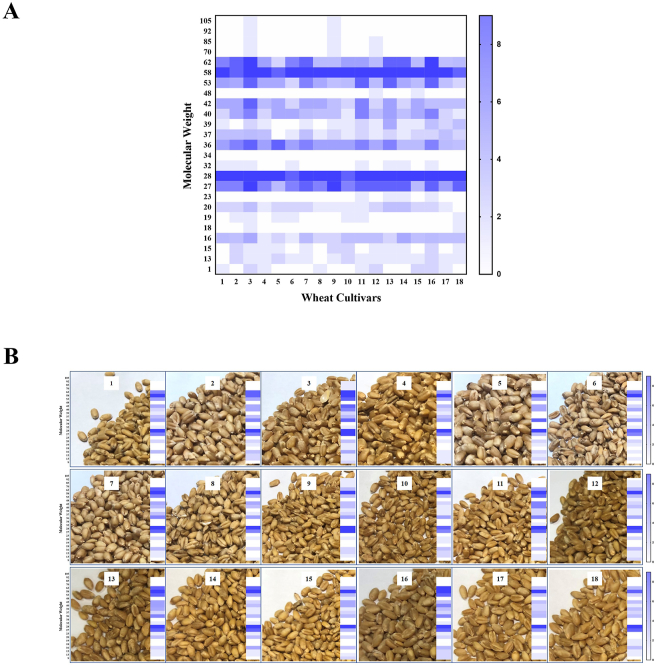
Firstly, we systematically analyzed and compared the allergen composition of different Chinese wheat cultivars through Western blot (Fig. 3) using ten positive sera. As shown in Fig. 3, we observed 5 IgE reactive bands in all cultivars, including 27, 28, 53, 58, and 62 kDa-proteins, indicating that the IgE binding capacity of wheat protein may be determined by these 5 gel bands. Additionally, patient No. 10 was weakly allergic to certain proteins with low molecular weight, ranging from 9 to 23 kDa. In the IgE-binding capacity measurement, all experimental groups (10 positive sera) exhibited very significant differences from the control group (Fig. 3), and all Western blot results were summarized in Fig. 4. It was obvious that 28 and 58 kDa-proteins had high allergenicity probability (>8/10), and such proteins with molecular weight at 27, 36, 40, 42, 53, and 62 kDa had moderate allergenicity probability (4/10–8/10), and 17 protein bands had low allergenicity probability (<4/10), including 9, 13, 15, 16, 18, 19, 20, 22, 23, 32, 34, 37, 39, 48, 70, 90, and 105 kDa-proteins. Furthermore, patients No. 2 and No. 7 recognized 28, 36, 40, 42, and 58 kDa-proteins, while patient No. 5 recognized 18, 28, and 58 kDa-proteins. These results indicate that 28, 58 kDa-proteins showed the highest IgE binding capacity, and followed by 27, 53, and 62 kDa-proteins, which might be the potential allergens in Chinese wheat. In addition, there was no significant difference between the allergen composition of Chinese wheat and the ethnicity of the patients. According to Fig. S1, interestingly, these 5 potential allergens accounted for more than a third (36.8%), and proteins having moderate and high allergenicity probability accounted for nearly half (47.22%). Secondly, we explored the correlation analysis between the protein contents and IgE binding capacity. The relative contents of 5 potential allergens (27, 28, 53, 58, and 62 kDa) of the 18 wheat cultivars were analyzed by SDS-PAGE (Table S2). Compared with the ELISA results, the IgE binding capacity of wheat cultivars showed a significant correlation with the relative abundances of allergen fractions in soluble protein (Table 1). Interestingly, 3 wheat cultivars that have gained significantly high IgE binding capacity, included Zheng 366 (No. 3), Huai 40 (No. 4), Luo 26 (No. 11). In these wheat cultivars, the relative abundances of 28 kDa-protein and 58 kDa-protein were slightly higher than that of other cultivars. The correlation coefficients for 28 kDa-protein and 58 kDa-protein, 0.656 (P = 0.003) and 0.792 (P = 0.0001) were also very significant and positive. However, the correlation coefficients with a total content of 5 potential allergens and the IgE binding capacity were not significant.
Fig. 3.
Western blot of soluble protein using positive sera (A to J) in 18 Chinese wheat cultivars (1–18). (Note: A, No.1 serum; B, No.2 serum; C, No.3 serum; D, No.4 serum; E, No.5 serum; F, No.6 serum; G, No.7 serum; H, No.8 serum; I, No.9 serum; J, No.10 serum; lane 1, Zhong 1211; lane 2, Zhou 32; lane 3, Zheng 366; lane 4, Huai 40; lane 5, Luo 29; lane 6, Yan 4110; lane 7, Bai 64; lane 8, Feng 20; lane 9, Bai 4199; lane 10, Zhou 18; lane 11, Luo 26; lane 12, Zhou 27; lane 13, Zhou 36; lane 14, Bai 58; lane 15, Zheng 113; lane 16, Zheng 136; lane 17, Bai 207; lane 18, Xu 918.)
Fig. 4.
Heat map of potential allergens (A) in 18 Chinese wheat cultivars (B). Different colors represented the number of positive sera. (Note: lane 1, Zhong 1211; lane 2, Zhou 32; lane 3, Zheng 366; lane 5, Huai 40; lane 5, Luo 29; lane 6, Yan 4110; lane 7, Bai 64; lane 8, Feng 20; lane 9, Bai 4199; lane 10, Zhou 18; lane 11, Luo 26; lane 12, Zhou 27; lane 13, Zhou 36; lane 14, Bai 58; lane 15, Zheng 113; lane 16, Zheng 136; lane 17, Bai 207; lane 18, Xu 918.)
Table 1.
Correlation analysis between relative abundances of gel bands and IgE binding capacity in different Chinese wheat cultivars.
| 27 kDa | 28 kDa | 53 kDa | 58 kDa | 62 kDa | Total (%) | IgE binding capacity | |
|---|---|---|---|---|---|---|---|
| 27 kDa | 1 | ||||||
| 28 kDa | −0.003 | 1 | |||||
| 53 kDa | 0.258 | −0.110 | 1 | ||||
| 58 kDa | −0.355 | 0.150 | −0.300 | ||||
| 62 kDa | 0.015 | 0.301 | −.0117 | −0.575a | 1 | ||
| Total (%) | 0.228 | 0.600b | 0.588a | 0.013 | 0.301 | 1 | |
| IgE binding capacity | −0.143 | 0.656b | −0.229 | 0.792b | −0.374 | 0.314 | 1 |
Significant correlation (P < 0.05).
Very significant correlation (P < 0.01)
Finally, the bands of the 27, 28, 53, 58, and 62 kDa-proteins were identified by LC-MS/MS (Fig. S2). As shown in Table S3, more than 1 protein was identified in each gel band. However, a search for homologous proteins in UniProt-wheat-Triticum aestivum database showed that 15 peptides from 27 kDa-protein matched LEA1 protein (Accession: Q8GV49) with 64.3% identity with aligned amino acid sequences, 15 peptides from 28 kDa-protein matched rRNA N-glycosidase (Accession: A0A3B6LWE2) with 70.3% coverage, 20 peptides from 53 kDa-protein matched dihydrolipoyl dehydrogenase (Accession: W5A874) with 48.8% identity, 34 peptides from 58 kDa-protein matched β-amylase (Accession: A0A3B6IYD4) with 73% coverage, and 24 peptides from 62 kDa-protein matched disulfide-isomerase (Accession: F8THZ8) with 43.6% coverage. We further applied immunology tools such as IEDB, IMED, and ABCpred online websites to predict IgE epitopes of rRNA N-glycosidase and β-amylase according to their amino acid sequences. As shown in Table S4, a total of 32 and 44 peptides were predicted as allergenic epitopes in rRNA N-glycosidase and β-amylase, respectively. Therefore, the IgE binding capacity of Chinese wheat was mainly determined by rRNA N-glycosidase and β-amylase in soluble protein.18
Discussion
Currently, several proteins have been identified as the major allergens in wheat; however, only a few Chinese wheat cultivars have been screened on allergenicity. In the current study, we first extracted 3 classes of proteins and analyzed the protein composition in 18 major Chinese wheat cultivars, including soluble protein, gliadin, and glutenin. The amount of soluble protein, gliadin, and glutenin from Chinese wheat is similar to that of western wheat.19,20 No significant differences in protein composition and relative abundances of soluble protein and glutenin were found among the wheat cultivars from different regions. Besides, the relative abundances of gliadin with molecular weights between 29 and 44.3 kDa were significantly different.
Secondly, we investigated the allergenicity of 3 classes of wheat proteins from Chinese wheat cultivars using positive sera from American volunteer patients who were allergic to wheat and had allergic rhinitis. Allergic rhinitis is a typical symptom of allergy, including a runny nose, sneezing, and itchy eyes, mainly due to inhalation of allergens. Baker's asthma is an occupational allergic disease caused by inhalation or prolonged exposure to flour.21 Interestingly, soluble protein exhibited the highest allergenicity, followed by gliadin and glutenin. Generally, gliadin and glutenin were considered as the main allergens responsible for WDEIA.22,23 According to previous reports, the main allergens causing baker's asthma were soluble proteins, including tetrameric heterologous α-amylase inhibitor chloroform/methanol-soluble CM17 protein (WTAI-CM17), α-amylase inhibitor 0.19, and lipid transfer protein.24, 25, 26 Besides, these results were supported by a previous study that the IgE-binding frequencies were found for αβ-gliadin (10%) and ω-gliadin (2.5%) in Baker's asthma.7 Hence, soluble protein may be the main allergens of Chinese wheat causing allergic rhinitis and baker's asthma in patients from the United States.
Thirdly, the allergen profile analysis of soluble protein was performed in Chinese wheat. In 25 protein bands of soluble protein, 5 protein bands showed significant allergenicity, including 27, 28, 53, 58, and 62 kDa. Among them, 28 and 58 kDa-protein had the highest allergenicity probability. To date, different wheat proteins have been speculated to be the main causes of baker's asthma in different western countries, including thioredoxin (Sweden),27 dimeric a-amylase inhibitors (German),28 and chloroform/methanol-soluble (CM) 17 protein (Spain).24 However, the bands of the 3 proteins (13.4 kDa, 13 kDa, and 15.96 kDa) in Chinese wheat exhibited low allergenicity, showing the potential allergens of Chinese wheat inducing allergic rhinitis were different from that of western countries. These results demonstrated soluble proteins with molecular weights between 27 kDa and 62 kDa were the most frequent IgE-binding proteins. Kumar et al also found major IgE binding proteins with molecular weights between 11 kDa and 65 kDa in Buchanania lanzan, implying proteins with lower and higher molecular weights had weak allergenicity.29
Finally, the relationship between the contents of 5 potential allergens and wheat allergenicity was explored. The total content of the 5 potential allergens had little effect on the potential allergenicity of Chinese wheat. The relative abundances of 28 kDa-protein and 58 kDa-protein were significantly positively correlated with the IgE binding of Chinese wheat cultivars, demonstrating both proteins were the main allergens causing allergic rhinitis. 28 kDa-protein and 58 kDa-protein were identified as heterologous mixtures, particularly containing rRNA N-glycosidase and β-amylase. According to the report linear epitopes were easily involved with allergic reactions during exposure to gastrointestinal food allergens.30 A total of 15 and 34 peptides were predicted as allergenic epitopes in rRNA N-glycosidase and β-amylase, implying that rRNA N-glycosidase and β-amylase had strong allergenicity. The β-amylase has been named Tri a 17 by WHO/IUIS (http://www.allergen.org/). However, rRNA N-glycosidase has not been registered as a wheat allergen, indicating that it may be a novel allergen in Chinese wheat.
Conclusion
In summary, this work evaluated the allergenicity of 18 Chinese wheat cultivars and explored the allergen profile of Chinese wheat in patients with allergic rhinitis. In Chinese wheat, 5 gel bands were regarded as important allergens in soluble protein, among which we speculate that rRNA N-glycosidase and β-amylase affected the IgE binding capacity of Chinese wheat. This is the first report of allergen profile analysis of different Chinese wheat cultivars, although further studies about molecular cloning, heterologous expression, purification, and characterization of novel allergen are required. In addition, the traditional denaturing gel electrophoresis is limited in the separation of individual proteins. Further studies about allergenicity assessment of particular proteins separated by two-dimensional gel electrophoresis may provide a solution. Given the relevance of rRNA N-glycosidase and β-amylase as main allergens of allergic rhinitis, new insights may be provided into the prevention of wheat allergy and development of hypoallergenic wheat products.
Abbreviations
IgE, immunoglobulin E; WDEIA, wheat-dependent exercise-induced anaphylaxis; WHO/IUIS, World Health Organization and International Union of Immunological Societies; HMW-GS, high molecular weight glutenin subunits; LMW-GS, low molecular weight glutenin subunits; PBS, phosphate-buffered saline; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; PVDF, polyvinylidene fluoride; BSA, bovine serum albumin; TBST, Tris-buffered Saline Tween-20; HRP, horseradish peroxidase; ECL, electrochemiluminescence; ELISA, enzyme-linked immunosorbent assay; LC-MS/MS, liquid chromatography-tandem mass spectrometry; DTT, dithiothreitol.
Consent for publication
All authors provided consent for publication.
Authorship contribution
Yanbo Wang designed experiments and wrote the manuscript. Junjie Weng designed experiments and performed experiments. Chengbo Zhu analyzed data and wrote the manuscript. Rong Ai prepared materials and performed experiments. Jinru Zhou edited the manuscript. Chong Wang analyzed data. Qing Chen contributed to the overall planning. Linglin Fu contributed to the overall planning, project conception, and edited the manuscript.
Availability of data and materials
All data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author upon request.
Ethics approval
The study was approved by Zhejiang Gongshang University Ethics Review Committee as it is part of a routine procedure in which no additional consent is required by law.
Funding
This study was financially supported by the State Key Research and Development Plan [2019YFC1605002] and the National Natural Science Foundation of China [31871735].
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We are appreciated all the patients who participated in this study.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100559.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Jia F., Zhang L., Chen L., Wang J., Qi B. Allergens in wheat: review. Adv Mater Res. 2012;518–523:5510–5513. [Google Scholar]
- 2.Nwaru B., Hickstein L., Panesar S., Roberts G., Muraro A., Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 3.Vierk K., Koehler K., Fein S., Street D. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504–1510. doi: 10.1016/j.jaci.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhuang Y., Ma T., Zhang B., Wang X. Prevalence of self-reported food allergy in six regions of Inner Mongolia, northern China: a population-based survey. Med Sci Mon Int Med J Exp Clin Res. 2018;24:1902–1911. doi: 10.12659/MSM.908365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veraverbeke W., Delcour J. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. CRC Crit Rev Food Technol. 2002;42:179–208. doi: 10.1080/10408690290825510. [DOI] [PubMed] [Google Scholar]
- 6.Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009;9:238–243. doi: 10.1097/ACI.0b013e32832aa5bc. [DOI] [PubMed] [Google Scholar]
- 7.Sander I., Rozynek P., Rihs H.P. Multiple wheat flour allergens and cross-reactive carbohydrate determinants bind IgE in baker's asthma. Allergy. 2011;66:1208–1215. doi: 10.1111/j.1398-9995.2011.02636.x. [DOI] [PubMed] [Google Scholar]
- 8.Lupi R., Masci S., Rogniaux H., Tranquet O., Brossard C. Assessment of the allergenicity of soluble fractions from GM and commercial genotypes of wheats. J Cereal Sci. 2014;60:179–186. [Google Scholar]
- 9.Hiroaki M., Eishin M., Arthur Sydney T. Identification of the IgE-binding epitope in omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. J Biol Chem. 2004;279:12135–12140. doi: 10.1074/jbc.M311340200. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama N., Ichise K., Katsube T. Identification of major wheat allergens by means of the Escherichia coli expression system. Eur J Biochem. 2010;255:739–745. doi: 10.1046/j.1432-1327.1998.2550739.x. [DOI] [PubMed] [Google Scholar]
- 11.Battais F., Courcoux P., Popineau Y., Kanny G., Moneret-Vautrin D.A., Denery-Papini S. Food allergy to wheat: differences in immunoglobulin E-binding proteins as a function of age or symptoms. J Cereal Sci. 2005;42:109–117. [Google Scholar]
- 12.Le T.A., Al K.M., Tan J.A. The clinical spectrum of omega-5-gliadin allergy. Intern Med J. 2016;46:710–716. doi: 10.1111/imj.13091. [DOI] [PubMed] [Google Scholar]
- 13.Jiang N., Yin J., Wen L., Li H. Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy Asthma Immunol Res. 2016;8:353–361. doi: 10.4168/aair.2016.8.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xian J.W., Wu Hai-Qiang, Jian X.L., Liu Z.G. Extraction,Isolation and immunological identification of allergens from Triticum aestivum and fagopyrum esculentum. J Triticeae Crops. 2008;28:779–803. [Google Scholar]
- 15.Zhili P., Yuanqi L., Zhilu A., Na W., Xinhua X., Biao S. Relationship between composition of gliadin and texture properties of quick-frozen dumpling skins based on different wheat varieties. Nongye Gongcheng Xuebao/Trans Chinese Soc Agric Eng. 2016;32:242–248. [Google Scholar]
- 16.Verbruggen I.M., Veraverbeke W.S., Vandamme A., Delcour J.A. Simultaneous isolation of wheat high molecular weight and low molecular weight glutenin subunits. J Cereal Sci. 1998;28:25–32. [Google Scholar]
- 17.Song T.W., Hong J.Y., Lee K.E. IgE reactivity to carbohydrate moieties of glycoproteins in wheat allergy. Allergy Asthma Proc. 2015;36:192–199. doi: 10.2500/aap.2015.36.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer G., Wieser S., Bogdos M.K. Three-dimensional structure of the wheat β-amylase Tri a 17, a clinically relevant food allergen. Allergy. 2019;74:1009–1013. doi: 10.1111/all.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battais F., Mothes T., Moneret-Vautrin D.A. Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy. 2015;60:815–821. doi: 10.1111/j.1398-9995.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu X., Wei Y., Wang C., Kovacs M.I.P. Quantitative assessment of protein fractions of Chinese wheat flours and their contribution to white salted noodle quality. Food Res Int. 2007;40:1–6. [Google Scholar]
- 21.Baur X., Posch A. Characterized allergens causing bakers' asthma. Allergy. 2010;53:562–566. doi: 10.1111/j.1398-9995.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann S.C., Fischer J., Eriksson C., Gref O.B., Jakob T. IgE detection to α/β/γ-gliadin and its clinical relevance in wheat-dependent exercise-induced anaphylaxis. Allergy. 2012;67:1457–1460. doi: 10.1111/all.12020. [DOI] [PubMed] [Google Scholar]
- 23.Morita E., Matsuo H., Chinuki Y., Takahashi H., DahlströM J., Akira T. Food-dependent exercise-induced anaphylaxis—importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis—. Allergol Int. 2009;58:493–498. doi: 10.2332/allergolint.09-RAI-0125. [DOI] [PubMed] [Google Scholar]
- 24.Ingrid Sander, Hans-Peter Rihs, Thomas Brüning, Monika Raulf. A further wheat allergen for baker's asthma: Tri a 40. J Allergy Clin Immunol. 2016;137:1286. doi: 10.1016/j.jaci.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Weichel M., Glaser A.G., Ballmer-Weber B.K., Schmid-Grendelmeier P., Crameri R. Wheat and maize thioredoxins: a novel cross-reactive cereal allergen family related to baker's asthma. J Allergy Clin Immunol. 2006;117:676–681. doi: 10.1016/j.jaci.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Palacin A., Quirce S., Armentia A. Wheat lipid transfer protein is a major allergen associated with baker's asthma. J Allergy Clin Immunol. 2007;120:1132–1138. doi: 10.1016/j.jaci.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Michael W., Glaser A.G., Ballmer-Weber B.K., Peter S.G., Reto C. Wheat and maize thioredoxins: a novel cross-reactive cereal allergen family related to baker's asthma. J Allergy Clin Immunol. 2006;117:676–681. doi: 10.1016/j.jaci.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 28.Sander I., Rihs H.P., Doekes G., Quirce S., Raulf M. Component-resolved diagnosis of baker's allergy based on specific IgE to recombinant wheat flour proteins. J Allergy Clin Immunol. 2015;135:1529–1537. doi: 10.1016/j.jaci.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S., Sharma A., Gupta R.K., Verma A.K., Dwivedi P D. Allergenicity assessment of Buchanania lanzan protein extract in Balb/c mice. Int Immunopharm. 2018;63:170–182. doi: 10.1016/j.intimp.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer S., Bublin M., Dubiela P. Cor a 14, the allergenic 2S albumin from hazelnut, is highly thermostable and resistant to gastrointestinal digestion. Molecular Nutrition Food Research International. 2015;59:2077–2086. doi: 10.1002/mnfr.201500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author upon request.