Fig. 7.
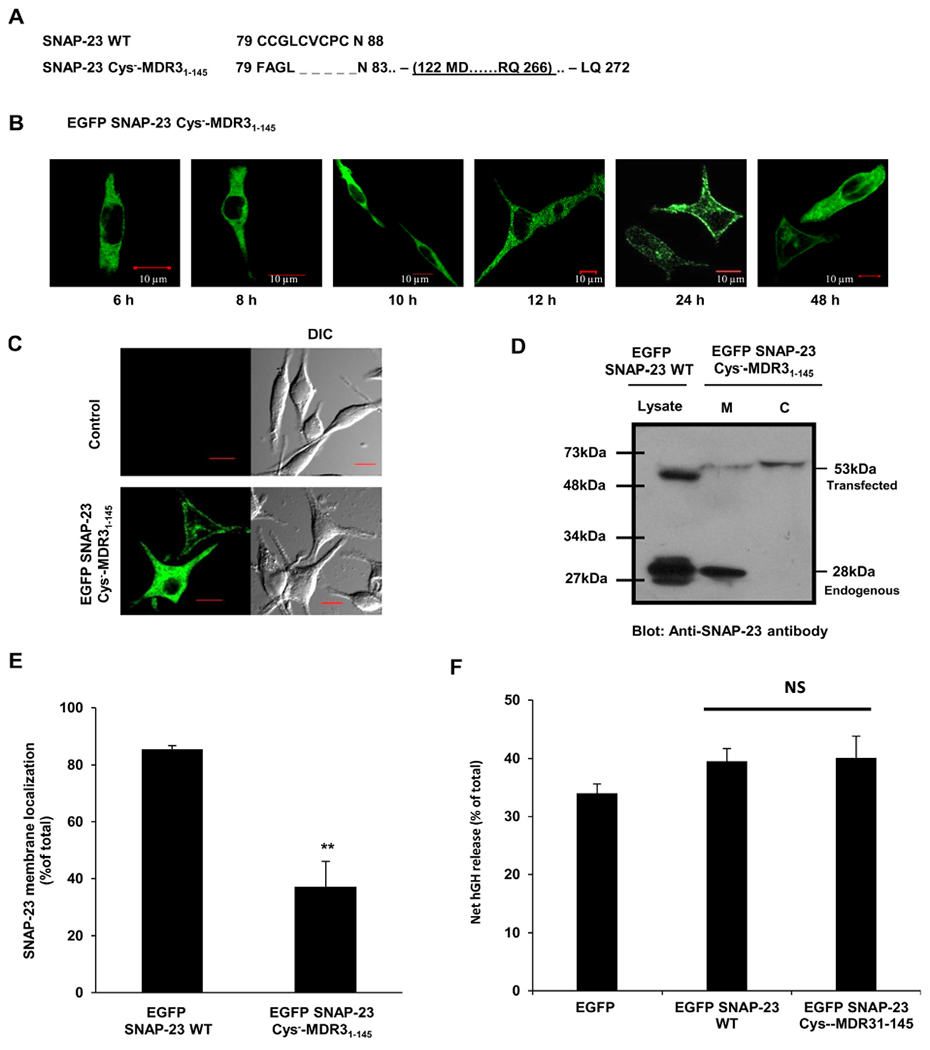
SNAP-23 Cys−-MDR31–145 transmembrane fusion protein shows delayed and only partial trafficking to the plasma membrane of RBL mast cells. A. Amino acid sequence of the cysteine-rich domain of SNAP-23 WT and of the corresponding region of SNAP-23 Cys−-MDR31–145 fusion construct. Also shown (underlined) is the cloning site of MDR31–145 domain in SNAP-23 Cys− mutant. B. RBL cells were transfected with EGFP-tagged SNAP-23 Cys−-MDR31–145 fusion construct. Transfected cells were plated on coverslips and analyzed by confocal microscopy after 6, 8, 10, 12, 24 and 48 h post-transfection. Shown here are representative images. Scale = 10 μm. C. RBL cells were either mock transfected (control) or transfected with EGFP-tagged SNAP-23 Cys−-MDR31–145 fusion construct. 48 h post-transfection the cells were analyzed by confocal microscopy. Shown here are representative images. Scale = 10 μm. D. Lysates and membrane/cytosol enrichment fractions from EGFP-tagged SNAP-23 Cys−-MDR31–145 fusion construct transfected RBL cells were analyzed by immunoblotting with anti-SNAP-23 antibody. Shown here is a representative immunoblot with position of molecular weight markers. E. Band intensities from three independent experiments were quantified by densitometry and expressed as SNAP-23% membrane localization with respect to total SNAP-23 (mean ± SEM). ** indicate statistically significant difference in membrane association of SNAP-23 Cys−-MDR31–145 fusion protein as compared to wild-type SNAP-23 (p < 0.005). F. Effect of SNAP-23 Cys−-MDR31–145 fusion protein in mast cell exocytosis was detected by hGH reporter assay. Each data point is a mean ± SEM of three independent experiments (ns = not significant).
