Fig. 9.
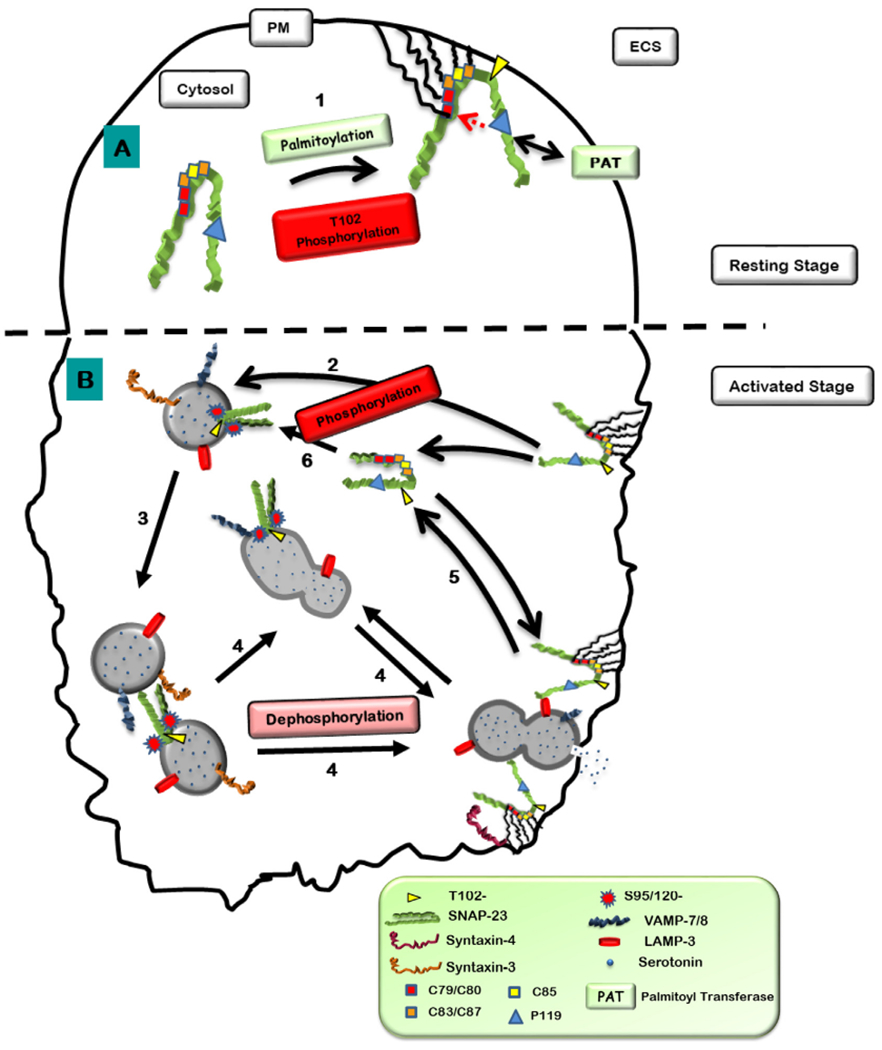
Hypothetical model showing hydrophobic interactions and palmitoylations of cysteine in the linker region of SNAP-23 regulate its membrane association. A. After synthesis in the cytosol, 1. SNAP-23 may initially associate with membrane (internal or plasma membrane) through some hydrophobic interactions due to presence of hydrophobic amino acids like cysteines in the linker region. Since DHHC palmitoyl transferases (PAT) which palmitoylate cysteine residues in proteins are localized on membranes, initial hydrophobic and electrostatic interaction with membrane may bring SNAP-23 close to these DHHC proteins which can now palmitoylate the conserved cysteine residues in SNAP-23 and thereby confine it to membrane. All cysteine residues except for C85 seemed to be important for membrane association of SNAP-23 in mast cells. P119 residue of SNAP-23 (corresponding P117 residue in SNAP-25 involved in the palmitoylation of cysteine residues in its linker region thereby in its membrane targeting) seems to be important for membrane association of SNAP-23 in mast cells and may be required for interaction with DHHC palmitoyl transferases. Apart from that it has also shown that the basal phosphorylation at T102 site also plays an important role in SNAP-23 initial plasma membrane association [22]. B. After Crosslinking of mast cells, 2. The phosphorylation of SNAP-23 at S95 and S120 sites mediates its relocation to internal LAMP-3 positive granule membranes, 3–4. There may be the phosphorylation helps in homotypic fusions (granule-granule fusions) as Syntaxin-3 present there (may play the cognitive t-SNARE of SNARE-tri-molecular complex, [22]). Then the dephosphorylation of these residues mediate the plasma membrane fusions of these granules (compound exocytosis) [21]. 5–6. Then SNAP-23 relocates to cytosol and phosphorylated and associates to LAMP-3 positive granule membrane.
