Abstract
Background:
Bilateral oophorectomy during a non-malignant hysterectomy is frequently performed for ovarian cancer prevention in premenopausal women. Oophorectomy before menopause leads to an abrupt decline in ovarian hormones that could adversely impact body composition. We examined the relationship between oophorectomy and whole-body composition.
Methods:
Our study population included cancer-free women 35–70 years old from the 1999–2006 National Health and Nutrition Examination Survey, a representative sample of the U.S. population. A total of 4,209 women with dual-energy x-ray absorptiometry scans were identified, including 445 with hysterectomy, 552 with hysterectomy and oophorectomy, and 3,212 with no surgery. Linear regression was used to estimate the difference in total and regional (trunk, arms, legs) fat and lean body mass by surgery status.
Results:
In multivariable models, hysterectomy with and without oophorectomy was associated with higher total fat mass (mean percent difference (β); βoophorectomy: 1.61%, 95% CI: 1.00, 2.28%; βhysterectomy: 0.88%, 95% CI: 0.12, 1.58) and lower total lean mass (βoophorectomy: −1.48%, 95% CI: −2.67, −1.15; βhysterectomy: −0.87%, 95% CI: −1.50, −0.24) compared to no surgery. Results were stronger in women with a normal BMI and those <45 years at surgery. All body regions were significantly affected for women with oophorectomy, while only the trunk was affected for women with hysterectomy alone.
Conclusions:
Hysterectomy with oophorectomy, particularly in young women, may be associated with systemic changes in fat and lean body mass irrespective of BMI.
Impact:
Our results support prospective evaluation of body composition in women undergoing hysterectomy with oophorectomy at a young age.
Keywords: Oophorectomy, hysterectomy, ovarian cancer, body composition, risk-reducing surgeries
INTRODUCTION
Many average-risk women undergo bilateral oophorectomy for ovarian cancer prevention at the time of a non-malignant hysterectomy. Hysterectomy is the most common non-obstetric gynecologic surgery in the U.S., with about 500,000 performed annually.1 Over 90% of all hysterectomies are performed for non-malignant gynecologic conditions such as uterine fibroids, abnormal uterine bleeding, and endometriosis. It is estimated that a bilateral oophorectomy is performed in 46%−52% of all hysterectomies, both for non-malignant gynecologic conditions and cancer prevention.2,3 Bilateral oophorectomy during a non-malignant hysterectomy is performed in 31% of women <45 years, 59% of women 45–49 years, and 74% of women ≥50 years.3 Additionally, women at high risk of ovarian cancer, such as BRCA½ carriers, are counseled to undergo prophylactic oophorectomy after childbearing or between 35 and 40 years.4 Despite the high prevalence of these surgeries, the long-term health effects of oophorectomy remain poorly understood.
Prior studies have found an increased incidence of cardiovascular disease,5,6 cognitive decline and dementia,7 and increased all-cause and cardiovascular disease mortality8,9 after oophorectomy. Oophorectomy, particularly in premenopausal women, has also been linked to increased adiposity, measured by body mass index (BMI), waist circumference, and skinfold thickness.10–12 To our knowledge, no prior studies have examined the association of oophorectomy and objectively measured total and regional fat and lean body mass.
Body fat is a more sensitive marker of adiposity, and it can vary considerably even among individuals with the same BMI or weight. Excess body fat is an established risk factor for cancer and cardiovascular disease independent of BMI.13–15 Additionally, there is growing evidence that adequate lean mass is beneficial for overall health and loss of lean mass or sarcopenia is associated with physical disability and mortality in older adults.16,17
Changes that occur during natural menopause, such as increased systemic inflammation,18,19 increased glucose and insulin levels,20 and increased pro-inflammatory cytokine levels,21,22 appear to be accelerated in women who undergo premenopausal oophorectomy due to the abrupt decline in endogenous estrogen and androgen. These hormonal changes have also been implicated in the gain of fat mass and loss of lean mass.23
The objective of this study was to examine the association of oophorectomy with total and regional fat and lean body mass measured using dual-energy x-ray absorptiometry (DXA) scans. DXA scans are considered the gold standard for body composition assessment and the most accurate way to measure fat and lean body mass.24–26 Since oophorectomy is commonly performed at the time of hysterectomy, we compared total and regional fat and lean body mass in women who reported hysterectomy with oophorectomy and hysterectomy alone to women with intact ovaries and uterus (no surgery).
METHODS
Study Population
Data were drawn from the National Health and Nutrition Examination Survey (NHANES), a population-based cross-sectional survey which uses stratified, multistage probability sampling to produce nationally representative estimates of the civilian, noninstitutionalized U.S. population. The protocols for the conduct of NHANES were approved by the Institutional Review Board of the National Center for Health Statistics, and all participants provided written informed consent prior to data collection. This study was deemed exempt by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health as the data used were deidentified. The study was conducted in accordance with recognized ethical guidelines (U.S. Common Rule).
The current study used four cycles of NHANES data from 1999 to 2006. The study sample was restricted to women 35–70 years old who completed both the interview and medical examination and had DXA measurements (n=5,225). A minimum age of 35 years was used as few women younger than 35 years undergo oophorectomy and women over 70 years were excluded because studies have shown a more rapid decline in lean mass after age 70.27,28
The reproductive health questionnaire was used to ascertain history of gynecologic surgery. Women were excluded if they were missing age at surgery (n=4), height and weight at DXA scan (n=170), or weight at age 25 (n=205). Women were also excluded if they reported removal of one ovary at the time of hysterectomy (n=299) and had oophorectomy without hysterectomy (n=57). Women with a history of reproductive cancers (breast, n=118; cervical, n=81; uterine, n=50; ovarian, n=32) were also excluded. After exclusions, 4,209 women were included in the analytic sample, 3,212 without surgery (intact ovaries and uterus), 445 with hysterectomy, and 552 with hysterectomy and oophorectomy. For brevity, oophorectomy will denote hysterectomy with bilateral oophorectomy for the rest of the manuscript.
Assessment of body composition
Whole-body DXA scans were used to ascertain total and regional fat and lean body mass. Scans were performed during the medical examination using a Hologic QDR 4500A fan beam x-ray bone densitometer (Hologic, Inc., Bedford, MA) and reviewed for quality control using Hologic Discovery software, version 12.1. About 20% of DXA scans were deemed invalid and coded as missing data. All missing data were imputed by NHANES using sequential regression multivariate imputation.29 Five data files containing both the non-missing and imputed DXA data were provided. Continuous measurements were provided for total and regional (arms, trunk, legs) fat mass and total and regional lean mass excluding bone mineral content.30 DXA values were also categorized into tertiles to classify women into low (T1), moderate (T2), and high (T3) fat and lean body mass. Tertile cut points were based on the distribution of fat and lean mass among women without surgery (Supplementary Table 1). A high total fat mass and low total lean mass body composition variable was created by jointly classifying women with high total fat mass (T3) and low total lean mass (T1).
Covariates
Information on demographics and lifestyle factors, including age, sex, race, education, income, smoking, physical activity, cigarette/tobacco use, weight history, and reproductive history was based on the in-person interview conducted before the medical examination. Alcohol consumption was categorized as ≥12 drinks in the past year or <12 drinks in the past year. Additionally, respondents were asked to estimate the average number of alcoholic drinks they consumed per day in the past year. Smoking was categorized as never, former, and current. Physical activity over the past month, including exercise, sports, and physically active hobbies was categorized as none, moderate, and vigorous. Moderate physical activity was defined as activities for at least 10 minutes that cause light sweating or slight to moderate increases in breathing or heart rate and vigorous physical activity was defined as activities for at least 10 minutes that cause heavy sweating or large increases in breathing or heart rate. Age at surgery was categorized as <45 and ≥45 years. This cut point was selected as most women were likely to be premenopausal at 4531 and to allow comparability with prior studies.5,8,32–34 Parity was classified as parous or nulliparous and post-surgery estrogen replacement therapy (ERT) use was classified as yes if women reported use of estrogen after surgery and no otherwise. History of endometriosis and uterine fibroids were classified based on prior physician diagnosis. Body weight and height were measured during the medical examination. BMI was calculated from measured height and weight and categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2).
Statistical analyses
Characteristics of women with and without surgery were compared using t-tests for continuous variables and chi-square tests for categorical variables. Linear regression models were used to estimate the mean difference in percent fat and lean body mass by surgery status. Distributions of total and regional fat and lean mass were examined graphically and no departures from normality were observed. Multinomial logistic regression models were utilized to estimate the odds ratio of being in the middle vs. the lowest tertile (T2 vs. T1) and the highest vs. the lowest tertile (T3 vs. T1) by surgery status. Results were reported overall and by age at surgery. Effect modification by age at DXA scan, race, and BMI at DXA scan was evaluated by adding cross product terms to the regression model. Age-adjusted prevalence of high total fat and low total lean body mass was calculated overall, by age at surgery, by physical activity, and by post-surgery ERT use. All analyses were weighted to account for the complex survey design and produce results generalizable to the U.S. population. Standard errors were obtained using the Taylor series (linearization) method.
Models were adjusted for age at DXA scan, race, income, physical activity, smoking, education, alcohol use, oral contraceptive use, BMI at age 25, and post-surgery ERT use. Sensitivity analyses were performed by additionally adjusting for total number of calories consumed from a 24-hour dietary recall, excluding women who reported post-surgery ERT use, excluding women with a diagnosis of uterine fibroids and endometriosis, and limiting the analysis to women with a history of hysterectomy with and without oophorectomy.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc.) and Stata, version 14.2 (Stata Corporation). Analyses were run separately on each imputed dataset provided by NHANES and the resulting estimates were combined to produce a single estimate.
RESULTS
Table 1 summarizes the characteristics of the 4,209 women by history of hysterectomy with and without and oophorectomy. Women with a history of surgery were older at DXA scan, less likely to have a college education, and more likely to have an annual family income of <$20,000 compared to women without surgery. Women with oophorectomy were more likely to be non-Hispanic white compared to women without surgery, whereas women with hysterectomy alone were more likely to be non-Hispanic Black. Women with a history of oophorectomy were older at the time of surgery compared to those with a history of hysterectomy alone. A higher proportion of women with surgery reported a diagnosis of endometriosis and uterine fibroids compared to women without surgery. There were no differences in parity, oral contraceptive use, alcohol consumption, and smoking between the groups. About 66% of women with oophorectomy and 22% of women with hysterectomy reported post-surgery ERT use. Women with a history of surgery were more likely to be obese at DXA scan and report no physical activity compared to women without surgery. BMI at age 25 (prior to surgery) did not differ between the groups.
Table 1.
Characteristics of the study population by hysterectomy and oophorectomy status
| No surgery (n=3,212) | Hysterectomy (n=445) | Hysterectomy & Oophorectomy (n=552) | P value | |
|---|---|---|---|---|
| Age at DXA scan, years, mean (SD) | 48.2 (7.2) | 53.8 (7.8) | 54.4 (7.4) | <0.001 |
| Age at surgery, years, mean (SD) | N/A | 38.3 (6.2) | 40.6 (6.2) | N/A |
| Race, % | ||||
| Non-Hispanic white | 71.4 | 71.0 | 80.8 | |
| Non-Hispanic black | 10.8 | 13.9 | 10.9 | |
| Mexican American | 6.3 | 4.0 | 2.9 | |
| Other | 11.5 | 11.1 | 5.4 | <0.001 |
| Highest education, % | ||||
| High school or less | 38.3 | 49.3 | 51.7 | |
| Some college or above | 57.9 | 48.5 | 45.4 | |
| Missing | 3.7 | 2.2 | 3.0 | <0.001 |
| Annual family income, % | ||||
| <$20,000 | 17.8 | 21.3 | 22.9 | |
| ≥$20,000 | 80.1 | 77.1 | 73.6 | |
| Missing | 2.1 | 1.6 | 3.5 | 0.024 |
| Smoking status, % | ||||
| Never | 56.0 | 54.5 | 48.9 | |
| Former | 22.9 | 24.6 | 23.6 | |
| Current | 21.1 | 20.9 | 27.5 | 0.073 |
| Parous, % | 94.9 | 94.2 | 97.9 | 0.126 |
| Diagnosed with endometriosis, % | 4.0 | 8.5 | 21.7 | <0.001 |
| Diagnosed with uterine fibroids, % | 9.7 | 22.6 | 24.6 | <0.001 |
| Oral contraceptive use, % | 72.0 | 74.7 | 74.9 | 0.423 |
| BMI at DXA scan, % | ||||
| Underweight | 1.7 | 0.7 | 1.6 | |
| Normal | 34.7 | 30.5 | 25.9 | |
| Overweight | 27.8 | 25.8 | 30.4 | |
| Obese | 35.8 | 43.0 | 42.1 | 0.020 |
| BMI at age 25, % | ||||
| Underweight | 8.9 | 9.2 | 10.8 | |
| Normal | 71.8 | 70.9 | 72.7 | |
| Overweight | 12.4 | 11 | 9.6 | |
| Obese | 6.9 | 8.9 | 6.9 | 0.268 |
| No. of drinks per week over past year, mean (SD) | 2.0 | 1.8 | 1.8 | 0.184 |
| Physical activity over past 30 days, % | ||||
| None | 36.2 | 45.6 | 40.2 | |
| Moderate | 32.2 | 34.5 | 38.1 | |
| Vigorous | 31.6 | 19.9 | 21.8 | <0.001 |
| Post-surgery estrogen replacement therapy, % | N/A | 21.9 | 66.4 | N/A |
Abbreviations: SD, standard deviation; DXA, dual-energy x-ray absorptiometry; N/A, not applicable.
Total fat and lean body mass
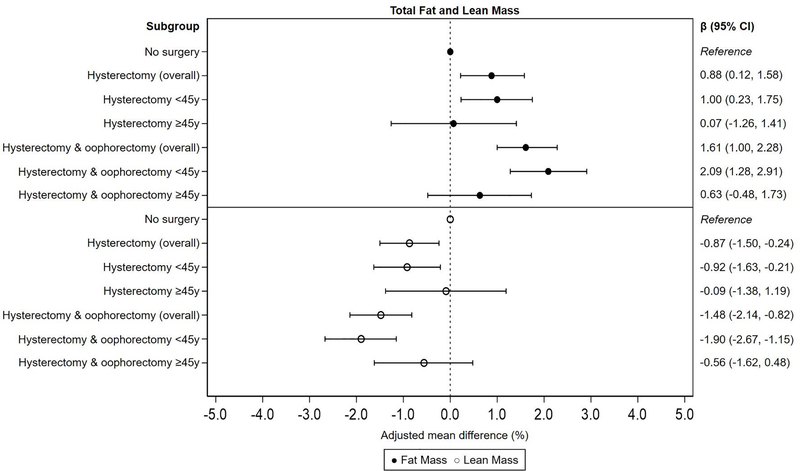
After adjusting for age, race, income, physical activity, smoking, education, alcohol use, oral contraceptive use, BMI at age 25, and post-surgery ERT use, women with oophorectomy had on average 1.61% (95% CI: 1.00%, 2.28%) higher total fat mass and 1.48% (95% CI: −2.14%, −0.82%) lower total lean mass compared to women without surgery. In models stratified by age at surgery, associations were stronger and only statistically significant in women who had oophorectomy <45 years (βfat: 2.09%, 95% CI: 1.28%, 2.91%; βlean: −1.90%, 95% CI: −2.67%, −1.15%). Similarly, women with hysterectomy alone had 0.88% (95% CI: 0.12%, 1.58%) higher total fat mass and 0.87% (95% CI: −1.50%, −0.24%) lower total lean mass compared to women without surgery. Associations were stronger and only statistically significant in women who had hysterectomy <45 years (βfat: 1.00%, 95% CI: 0.23%, 1.75%; βlean: −0.92%, 95% CI: −1.63%, −0.21%; Figure 1).
Figure 1.
Multivariable-adjusted mean percent difference and 95% CI for total fat and lean mass by hysterectomy and oophorectomy status, overall and by age at surgery. Adjusted for age at interview, race, smoking, alcohol use, education, BMI at age 25, parity, oral contraceptive use, and post-surgery estrogen replacement therapy use.
Next, we evaluated the impact of age (<45 years, ≥45 years) and BMI (<25 kg/m2, ≥25 kg/m2) at DXA scan on the association between hysterectomy with and without oophorectomy and total fat and lean mass. We did not evaluate race as it was not a significant effect measure modifier. Among women <45 years at DXA scan, the median time since surgery was 6 years (interquartile range [IQR]: 2–10 years) for oophorectomy and 5 years (IQR: 2–11 years) for hysterectomy alone. Among women ≥45 years at DXA scan, the median time since surgery was 16 years (IQR: 7–23 years) for oophorectomy and 19 years (IQR: 12–26 years) for hysterectomy alone. Regardless of age at DXA scan, oophorectomy was associated with a significant increase in total fat mass and decrease in total lean mass with the greatest difference in the subgroup of women <45 years at DXA scan (βfat: 3.25%, 95% CI: 1.03%, 5.54%; βlean: −2.86%, 95% CI: −5.00%, −1.07%). A similar pattern was noted for hysterectomy alone, although the beta estimates were smaller when compared to oophorectomy (βfat: 1.33%, 95% CI: 0.00%, 2.66%; βlean: −1.22%, 95% CI: −2.47%, 0.03%; Table 2).
Table 2.
Multivariable-adjusted mean percent difference and 95% CI for total and regional fat and lean body mass by hysterectomy and oophorectomy status and age at DXA scan*
| DXA Measure | Subgroup | Age <45 y (n=1,365) |
Age ≥45 y (n=2,844) |
||||
|---|---|---|---|---|---|---|---|
| n | β (95% CI) | P value | n | β (95% CI) | P value | ||
| Total Fat Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | 1.33 (0.00, 2.66) | 0.051 | 377 | 0.76 (0.02, 1.51) | 0.046 | |
| Hysterectomy & oophorectomy | 70 | 3.25 (1.03, 5.54) | 0.005 | 482 | 1.29 (0.56, 2.01) | <0.001 | |
| Total Lean Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | −1.22 (−2.47, 0.03) | 0.055 | 377 | −0.73 (−1.42, −0.02) | 0.041 | |
| Hysterectomy & oophorectomy | 70 | −2.86 (−5.00, −1.07) | 0.009 | 482 | −1.20 (−1.87, −0.53) | <0.001 | |
| Trunk Fat Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | 1.76 (−0.04, 3.56) | 0.056 | 377 | 1.29 (0.23, 2.36) | 0.017 | |
| Hysterectomy & oophorectomy | 70 | 4.79 (2.08, 7.50) | <0.001 | 482 | 1.72 (0.78, 2.67) | <0.001 | |
| Trunk Lean Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | −1.70 (−3.46, 0.06) | 0.061 | 377 | −1.26 (−2.30, −0.20) | 0.016 | |
| Hysterectomy & oophorectomy | 70 | −4.56 (−7.20, −1.92) | <0.001 | 482 | −1.67 (−2.60, −0.74) | <0.001 | |
| Arms Fat Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | 1.33 (−0.41, 3.03) | 0.135 | 377 | 0.58 (−0.40, 1.56) | 0.242 | |
| Hysterectomy & oophorectomy | 70 | 3.56 (0.75, 6.37) | 0.013 | 482 | 1.13 (0.20, 2.14) | 0.029 | |
| Arms Lean Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | −1.11 (−2.76, 0.53) | 0.184 | 377 | −0.52 (−1.43, 0.39) | 0.259 | |
| Hysterectomy & oophorectomy | 70 | −3.07 (−5.72, −0.43) | 0.023 | 482 | −1.04 (−1.99, −0.09) | 0.033 | |
| Legs Fat Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | 0.95 (−0.53, 2.43) | 0.208 | 377 | 0.14 (−0.54, 0.82) | 0.691 | |
| Hysterectomy & oophorectomy | 70 | 1.37 (−0.97, 3.70) | 0.252 | 482 | 0.87 (0.20, 1.52) | 0.011 | |
| Legs Lean Mass | No surgery | 1,227 | Reference | 1,985 | Reference | ||
| Hysterectomy | 68 | −0.85 (−2.25, 0.54) | 0.231 | 377 | −0.16 (−0.81, 0.50) | 0.639 | |
| Hysterectomy & oophorectomy | 70 | −1.07 (−3.28, 1.14) | 0.343 | 482 | −0.80 (−1.43, −0.18) | 0.012 | |
Abbreviations: DXA, dual-energy x-ray absorptiometry; CI, confidence interval.
Median (interquartile range) time since hysterectomy and oophorectomy, <45 years: 6 years (2–10 years) and ≥45 years: 16 years (7–23 years); Median time since hysterectomy, <45 years: 5 years (2–11 years) and ≥45 years: 19 years (12–26 years). Adjusted for age, race, smoking, alcohol use, physical activity, BMI at age 25, parity, oral contraceptive use, and post-surgery estrogen replacement therapy use.
The association between oophorectomy and total fat and lean mass persisted regardless of BMI at DXA scan. Of note, the increase in fat mass and decrease in lean mass by oophorectomy history was stronger in women with BMI <25 kg/m2 at DXA scan. There was no statistically significant association between hysterectomy alone and total fat and lean mass in models stratified by BMI at DXA scan (Table 3).
Table 3.
Multivariable-adjusted mean percent difference and 95% CI for total and regional fat and lean body mass by hysterectomy and oophorectomy status and body mass index at DXA scan*
| DXA Measure | Subgroup | BMI <25 kg/m2 (n=1,177) |
BMI ≥25 kg/m2 (n=2,980) |
||||
|---|---|---|---|---|---|---|---|
| N | β (95% CI) | P value | n | β (95% CI) | P value | ||
| Total Fat Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | 1.00 (−0.19, 2.20) | 0.111 | 339 | 0.55 (−0.15, 1.26) | 0.125 | |
| Hysterectomy & oophorectomy | 132 | 1.89 (0.72, 3.04) | 0.002 | 415 | 0.75 (0.27, 1.23) | 0.002 | |
| Total Lean Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | −0.95 (−2.10, 0.20) | 0.105 | 339 | −0.51 (−1.18, 0.16) | 0.138 | |
| Hysterectomy & oophorectomy | 132 | −1.73 (−2.85, −0.60) | 0.003 | 415 | −0.70 (−1.15, −0.24) | 0.003 | |
| Trunk Fat Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | 1.32 (−0.17, 2.80) | 0.082 | 339 | 1.09 (0.20, 1.99) | 0.018 | |
| Hysterectomy & oophorectomy | 132 | 2.56 (1.14, 3.96) | <0.001 | 415 | 1.15 (0.51, 1.79) | <0.001 | |
| Trunk Lean Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | −1.25 (−2.71, 0.19) | 0.089 | 339 | −0.32 (−0.73, 0.09) | 0.123 | |
| Hysterectomy & oophorectomy | 132 | −2.45 (−3.82, −1.06) | <0.001 | 415 | −0.40 (−0.68, −0.12) | 0.006 | |
| Arms Fat Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | 1.04 (−0.69, 2.75) | 0.237 | 339 | 0.31 (−0.62, 1.24) | 0.512 | |
| Hysterectomy & oophorectomy | 132 | 1.82 (0.18, 3.46) | 0.031 | 415 | 0.72 (−0.05, 1.48) | 0.066 | |
| Arms Lean Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | −0.93 (−2.58, 0.73) | 0.271 | 339 | −0.24 (−1.12, 0.64) | 0.591 | |
| Hysterectomy & oophorectomy | 132 | −1.65 (−3.25, −0.04) | 0.044 | 415 | −0.65 (−1.40, 0.09) | 0.085 | |
| Legs Fat Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | 0.80 (−0.76, 2.35) | 0.313 | 339 | −0.20 (−0.99, 0.61) | 0.641 | |
| Hysterectomy & oophorectomy | 132 | 1.37 (−0.05, 2.78) | 0.059 | 415 | 0.21 (−0.46, 0.88) | 0.544 | |
| Legs Lean Mass | No surgery | 942 | Reference | 2,226 | Reference | ||
| Hysterectomy | 103 | −0.83 (−2.35, 0.69) | 0.283 | 339 | 0.21 (−0.56, 0.97) | 0.593 | |
| Hysterectomy & oophorectomy | 132 | −1.23 (−2.61, 0.15) | 0.081 | 415 | −0.19 (−0.83, 0.46) | 0.563 | |
Abbreviations: DXA, dual-energy x-ray absorptiometry; CI, confidence interval. Adjusted for age, race, smoking, alcohol use, physical activity, BMI at age 25, parity, oral contraceptive use, and post-surgery estrogen replacement therapy use.
Regional fat and lean body mass
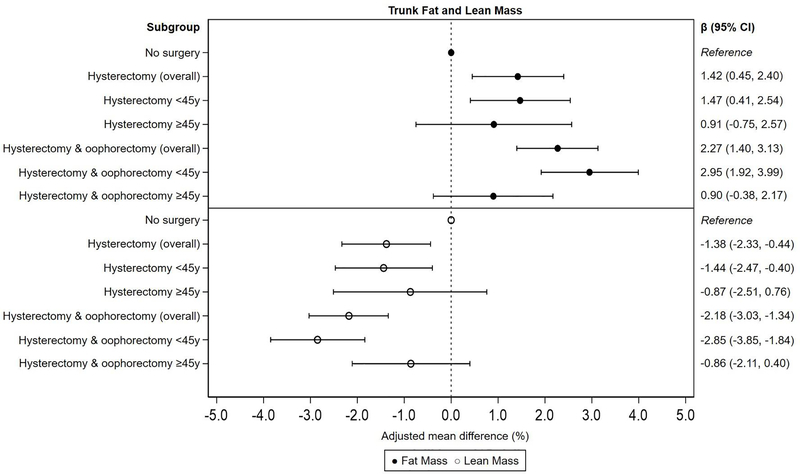
Women with oophorectomy had on average 2.27% (95% CI: 1.40%, 3.13%) higher trunk fat mass and 2.18% (95% CI: −3.03%, −1.34%) lower trunk lean mass compared to women without surgery, with stronger associations among women with oophorectomy <45 years (βfat: 2.95%, 95% CI: 1.92%, 3.99%; βlean: −2.85%, 95% CI: −3.85%, −1.84%). Women with hysterectomy had 1.42% (95% CI: 0.45%, 2.40%) higher trunk fat mass and 1.38% (95% CI: −2.33%, −0.44%) lower trunk lean mass compared to women without surgery, with stronger associations among women with hysterectomy <45 years (βfat: 1.47%, 95% CI: 0.41%, 2.54%; βlean: −1.44%, 95% CI: −2.47%, −0.40%; Figure 2). Significant differences in arms and legs fat and lean mass were only observed for women with oophorectomy (Supplementary Figure 1 and 2).
Figure 2.
Multivariable-adjusted mean percent difference and 95% CI for trunk fat and lean mass by hysterectomy and oophorectomy status, overall and by age at surgery. Adjusted for age at interview, race, smoking, alcohol use, education, BMI at age 25, parity, oral contraceptive use, and post-surgery estrogen replacement therapy use.
In analyses stratified by age at DXA scan, the increase in regional fat mass and decrease in regional lean mass was stronger in the subgroup of women <45 years at DXA scan (Table 2). In analyses stratified by BMI at DXA scan, the association between oophorectomy and regional fat and lean mass was stronger in women with BMI <25 kg/m2 at DXA scan. A similar pattern was noted for hysterectomy alone with respect to trunk fat and lean mass, although the beta estimates were smaller when compared to oophorectomy. There was no statistically significant association between hysterectomy alone and arms and legs fat and lean mass in BMI stratified analyses (Table 3).
Sensitivity analyses
Results were unchanged with additional adjustment for total caloric intake, and when women who reported post-surgery ERT use and women with a diagnosis of endometriosis and uterine fibroids were excluded (Supplementary Table 2). In analyses limited to women with a history of prior surgery, no significant differences were noted in total and regional fat and lean body mass in women with oophorectomy compared to women with hysterectomy alone (Supplementary Table 3). Results were similar for multinomial logistic regression models based on tertile cut points (Supplementary Table 4).
Prevalence of high total fat and low total lean body mass
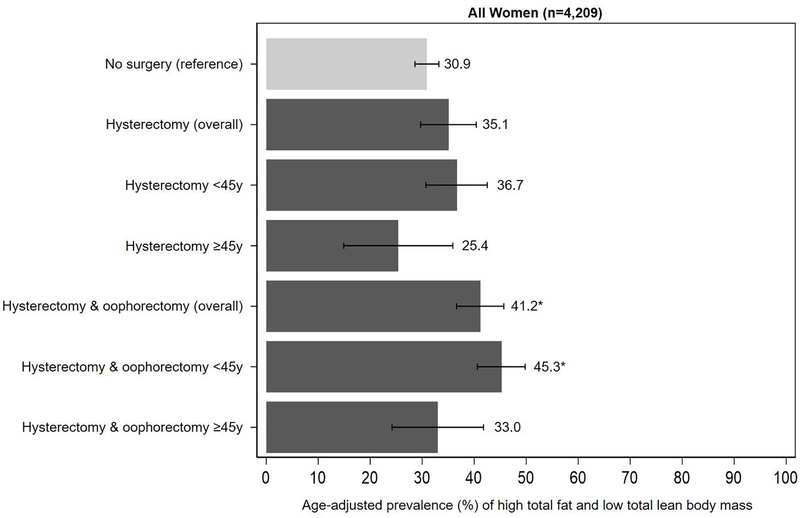
The age-adjusted prevalence of high total fat and low total lean body mass by hysterectomy and oophorectomy status is shown in Figure 3. Compared to women without surgery (30.9%, 95% CI: 28.6%, 33.2%), the prevalence of high total fat and low total lean mass was higher in women with oophorectomy (41.2%, 95% CI: 36.6%, 45.7%) and hysterectomy alone (35.1%, 95% CI: 29.7%, 40.4%), particularly those <45 years at the time of surgery (oophorectomy, 45.3%, 95% CI: 40.6%, 49.8%; hysterectomy alone, 36.7%, 95% CI: 30.7%, 42.5%).
Figure 3.
Age-adjusted prevalence of high total fat and low total lean body mass by hysterectomy and oophorectomy status, overall and by age at surgery. High total fat mass and low total lean body mass defined as total fat mass ≥43.7% (tertile 3) and total lean mass <53.7% (tertile 1). Tertile cut points are based on values among women without a history of hysterectomy and oophorectomy.
*P <0.05 for absolute differences in age-adjusted prevalence (reference group: no surgery).
Among women with a history of oophorectomy, a significantly lower prevalence of high total fat and low total lean mass was observed in women who reported vigorous physical activity (32.3%, 95% CI: 22.1%, 42.4%) compared to those who reported no physical activity (54.7%, 95% CI: 49.4%, 60.1%; p<0.001). Women who reported moderate physical activity (43.2%, 95% CI: 35.5%, 50.8%) also had a lower prevalence of high total fat and low total lean mass compared to women who reported no physical activity (54.7%, 95% CI: 49.4%, 60.1%); however, the difference was not statistically significant. Among women with a history of hysterectomy alone, a non-significant reduction in the prevalence of high total fat and low total lean mass was observed in women who reported vigorous physical activity (32.0%, 95% CI: 17.7%, 46.3%) and women who reported moderate physical activity (39.6%, 95% CI: 31.4%, 47.8%) compared to women who reported no physical activity (40.5%, 95% CI: 32.9%, 48.1%). A non-significant reduction in the prevalence of high total fat and low total lean mass was observed in women who reported ERT use after oophorectomy (44.4%, 95% CI: 29.5%, 49.4%) compared to those who did not (47.8%; 95% CI: 29.5%, 49.4%;). There was no difference in the prevalence of high total fat and low total lean mass in women who reported ERT use after hysterectomy (39.2%; 95% CI: 25.0%, 53.4%) compared to those who did not (38.4%, 95% CI: 32.5%, 44.3%; Supplementary Table 5).
DISCUSSION
To our knowledge, this is the first population-based study to compare DXA measurements of fat and lean body mass in women with a history of hysterectomy with and without oophorectomy. We found that women who had either surgery at a young age had increased total fat mass and decreased total lean mass relative to women with no surgery. However, differences in total fat and lean mass relative to women with intact ovaries and uterus were larger in women with a history of hysterectomy and oophorectomy than women with hysterectomy alone. All body regions (arms, legs, trunk) were affected for women with hysterectomy and oophorectomy, whereas only the trunk was affected for women with hysterectomy alone. Differences in total and regional fat and lean body mass by oophorectomy status were not limited to women who were overweight or obese but also observed in women with a normal BMI. Furthermore, these differences persisted after excluding women diagnosed with endometriosis and uterine fibroids and those using ERT after surgery. The age-adjusted prevalence of high total fat and low total lean mass was elevated among women with a history of surgery, with 45% of women with hysterectomy and oophorectomy <45 years and 37% of women with hysterectomy <45 years in the top third of total fat mass and the bottom third of total lean mass compared to 30% of women with no surgery.
Similar elevations in total body fat mass and trunk fat mass have been associated with increased cardiovascular disease and breast cancer risk. In the Women’s Health Initiative, high trunk fat mass was associated with a 1.9-fold increase in cardiovascular disease risk and a 1.8-fold increase in breast cancer risk in postmenopausal women with a normal BMI.13,15 Another study, investigating the association between body composition and overall mortality found that women in the lowest quartile of lean body mass had between 1.5- to 2.0-fold increased risk of overall mortality compared to women in other quartiles.35 Declines in lean body mass have also been linked to physical disability and frailty. A prospective study found that women with sarcopenia (severe loss of lean mass) had 1.5- to 2.8-times higher odds of physical disability when considering activities of daily living such as walking, bathing/dressing, and lifting/carrying.48 Another study examining the association between sarcopenia and all-cause mortality found that adults with sarcopenia had higher risk of all-cause mortality compared to adults without sarcopenia, regardless of BMI.49
The few prior studies that have assessed the association between hysterectomy with and without oophorectomy and adiposity have relied on indirect measures such as BMI,11,12,36–38 waist circumference,10,36 and skinfold thickness10 and have had small sample sizes. A prospective study found that the annual rate of increase in BMI for women with oophorectomy (n=106) was greater compared to women who underwent natural menopause (0.21 kg/m2 vs. 0.08 kg/m2, p=0.030) and women with oophorectomy (n=97) had three-times higher odds (95% CI: 1.48–6.72) of severe obesity (BMI ≥ 35 kg/m2) compared to women with natural menopause.11,12 Of note, age at oophorectomy in these prior studies was substantially higher than our study (45.7 years vs. 40.6 years). For hysterectomy alone, previous studies have found that women with a history of hysterectomy (n=76–236) had slightly higher post-surgery BMI compared to women with no hysterectomy (between 0.13–0.96 kg/m2)12,36,38 and women with hysterectomy had two-times higher odds (95% CI: 1.04–2.48) of reporting >10-pound weight gain compared to women with no hysterectomy.38 Our results suggest that BMI assessment alone is insufficient as it may mask important differences in fat and lean body mass in women with a normal BMI.
Our findings in younger women may be explained in part by the premature decline in sex hormones after surgery. During natural menopause, production of estrogen and progesterone decline gradually while production of testosterone continues.39,40 However, oophorectomy before natural menopause causes an abrupt decline in estrogen, progesterone, testosterone, and disruption of the hypothalamic-pituitary-ovarian axis.41,42 This disturbed hormonal milieu is thought to be a contributor to increased body weight, abdominal fat distribution, and decreased lean mass.43–45 Prior studies have suggested that hysterectomy alone could also result in hormonal changes by disrupting ovarian blood supply.46,47 Furthermore, changes in fat and muscle tissue in the abdomen could occur during an abdominal hysterectomy, which may explain why the greatest differences in fat and lean mass in our study were observed in the trunk region. While preclinical models of estrogen supplementation have been shown to counteract these changes,48–51 our results did not change when women who used post-surgery estrogen were excluded. The Women’s Health Initiative sub studies reported similar findings. They observed no significant differences in DXA-measured body composition in women randomized to estrogen therapy alone compared to placebo.52 Additionally, a recent systematic review showed no significant association between estrogen supplementation and muscle mass retention in postmenopausal women.53
Our study is subject to some limitations. Due to the cross-sectional design of NHANES, we were unable to assess causality between hysterectomy with and without oophorectomy and body composition. Overweight and obese women are more likely to be diagnosed with certain non-malignant gynecologic conditions such as uterine fibroids and abnormal menstrual bleeding compared to normal weight women, which may increase their likelihood of gynecologic surgeries. To address this potential bias, we stratified our analyses by BMI at DXA scan and conducted sensitivity analyses excluding women with a diagnosis of endometriosis and uterine fibroids. The relationship between oophorectomy and body composition persisted in BMI stratified analysis and was stronger in women with a normal BMI at DXA scan and our results were unchanged after exclusion of women with endometriosis and uterine fibroids. Additionally, we did not find a significant difference in BMI at age 25 between women who reported a subsequent surgery compared to women without surgery. Nevertheless, we adjusted for BMI at age 25 in all our multivariable models. Another limitation was that surgery status was self-reported and subject to recall bias. Studies that have investigated agreement of self-reported oophorectomy status indicate that accuracy exceeds 84% irrespective of age at surgery, hysterectomy status, and number of ovaries removed during surgery.54,55 Lastly, we cannot be certain that all oophorectomies were opportunistic. We excluded women with a cancer diagnosis from our study population, and our results were unchanged after excluding women who reported a diagnosis of uterine fibroids and endometriosis. Further, prior studies have shown that most bilateral oophorectomies during a non-malignant hysterectomy in premenopausal women are performed for ovarian cancer prevention.56
Our study has several strengths. First, NHANES provides a diverse sample of women representative of the U.S population with standardized protocols for questionnaires, interviewing, and examinations. Second, body composition was objectively measured using DXA scans, a technique that provides accurate, precise, and highly reproducible measures of fat and lean body mass.25,26 Finally, detailed information on demographic and reproductive factors allowed us to control for multiple important confounders.
In conclusion, our results suggest that hysterectomy with and without oophorectomy at a young age may have adverse effects on total fat and lean body mass, even among women with a normal BMI. The magnitude and extent of changes in fat and lean body mass may be greater in women who undergo hysterectomy with oophorectomy than hysterectomy alone. Prospective studies are needed to further evaluate body composition changes in women undergoing oophorectomy and the assessment of preventive interventions.
Supplementary Material
Acknowledgments
Financial support: NCI T32CA009314 Cancer Epidemiology, Prevention, and Control T32 Training Grant (Karia); The Breast Cancer Research Foundation (Visvanathan)
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Morgan DM, Kamdar NS, Swenson CW, Kobernik EK, Sammarco AG, Nallamothu B. Nationwide trends in the utilization of and payments for hysterectomy in the United States among commercially insured women. Am J Obstet Gynecol. 2018;218(4):425 e421–425 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacoby VL, Vittinghoff E, Nakagawa S, et al. Factors associated with undergoing bilateral salpingo-oophorectomy at the time of hysterectomy for benign conditions. Obstet Gynecol. 2009;113(6):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726. [DOI] [PubMed] [Google Scholar]
- 4.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–1110. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. [DOI] [PubMed] [Google Scholar]
- 8.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121(4):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy AM, Menke A, Ouyang P, Visvanathan K. Bilateral oophorectomy, body mass index, and mortality in U.S. women aged 40 years and older. Cancer Prev Res (Phila). 2012;5(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy AM, Menke A, Visvanathan K. Association of bilateral oophorectomy and body fatness in a representative sample of US women. Gynecol Oncol. 2013;129(3):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton-Tyrrell K, Zhao X, Santoro N, et al. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171(11):1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson CJ, Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Matthews KA. Body mass index following natural menopause and hysterectomy with and without bilateral oophorectomy. Int J Obes (Lond). 2013;37(6):809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyengar NM, Arthur R, Manson JE, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staunstrup LM, Nielsen HB, Pedersen BK, et al. Cancer risk in relation to body fat distribution, evaluated by DXA-scans, in postmenopausal women - the Prospective Epidemiological Risk Factor (PERF) study. Sci Rep. 2019;9(1):5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GC, Arthur R, Iyengar NM, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40(34):2849–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Taha M, Rius C, Hermenegildo C, et al. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183(2):1393–1402. [DOI] [PubMed] [Google Scholar]
- 19.Cioffi M, Esposito K, Vietri MT, et al. Cytokine pattern in postmenopause. Maturitas. 2002;41(3):187–192. [DOI] [PubMed] [Google Scholar]
- 20.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. [DOI] [PubMed] [Google Scholar]
- 21.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. [DOI] [PubMed] [Google Scholar]
- 22.Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88(12):5134–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9(4):186–197. [PubMed] [Google Scholar]
- 24.Borga M, West J, Bell JD, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fosbol MO, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Funct Imaging. 2015;35(2):81–97. [DOI] [PubMed] [Google Scholar]
- 26.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring). 2012;20(1):30–39. [DOI] [PubMed] [Google Scholar]
- 27.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–1007. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 29.Ragunathan TE, Lepkowski JV, Solenberger P. A multivariate technique for mulitply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27(2):85–95. [Google Scholar]
- 30.National Center for Health Statistics, NHANES: Technical documentation for the 1999–2004 dual energy x-ray absorptiometry (DXA) multiple imputation. 2008; https://wwwn.cdc.gov/Nchs/Nhanes/Dxa/Dxa.aspx. Accessed April 28, 2020.
- 31.Nichols HB, Trentham-Dietz A, Hampton JM, et al. From menarche to menopause: trends among US Women born from 1912 to 1969. Am J Epidemiol. 2006;164(10):1003–1011. [DOI] [PubMed] [Google Scholar]
- 32.Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta-Analysis. J Am Heart Assoc. 2016;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113(5):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc. 2016;91(11):1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toss F, Wiklund P, Nordstrom P, Nordstrom A. Body composition and mortality risk in later life. Age Ageing. 2012;41(5):677–681. [DOI] [PubMed] [Google Scholar]
- 36.Cooper R, Kuh D, Hardy R, Power C. Is there an association between hysterectomy and subsequent adiposity? Maturitas. 2007;58(3):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald DM, Berecki-Gisolf J, Hockey RL, Dobson AJ. Hysterectomy and weight gain. Menopause. 2009;16(2):279–285. [DOI] [PubMed] [Google Scholar]
- 38.Moorman PG, Schildkraut JM, Iversen ES, et al. A prospective study of weight gain after premenopausal hysterectomy. J Womens Health (Larchmt). 2009;18(5):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39(3):361–365. [DOI] [PubMed] [Google Scholar]
- 41.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26(41):10332–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas. 2011;68(4):331–336. [DOI] [PubMed] [Google Scholar]
- 44.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric. 2006;9(4):283–289. [DOI] [PubMed] [Google Scholar]
- 47.Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118(6):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP. Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci. 1999;64(24):2299–2306. [DOI] [PubMed] [Google Scholar]
- 50.Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol. 2010;122(1–3):100–105. [DOI] [PubMed] [Google Scholar]
- 51.Mattace Raso G, Irace C, Esposito E, et al. Ovariectomy and estrogen treatment modulate iron metabolism in rat adipose tissue. Biochem Pharmacol. 2009;78(8):1001–1007. [DOI] [PubMed] [Google Scholar]
- 52.Bea JW, Zhao Q, Cauley JA, et al. Effect of hormone therapy on lean body mass, falls, and fractures: 6-year results from the Women’s Health Initiative hormone trials. Menopause. 2011;18(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javed AA, Mayhew AJ, Shea AK, Raina P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2(8):e1910154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin KL, Wingo PA, Lee NC. Agreement of self-reported ovarian number following gynecologic surgery with medical record reports. J Clin Epidemiol. 1990;43(2):181–187. [DOI] [PubMed] [Google Scholar]
- 55.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. [DOI] [PubMed] [Google Scholar]
- 56.Karp NE, Fenner DE, Burgunder-Zdravkovski L, Morgan DM. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstet Gynecol. 2015;213(5):716 e711–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.