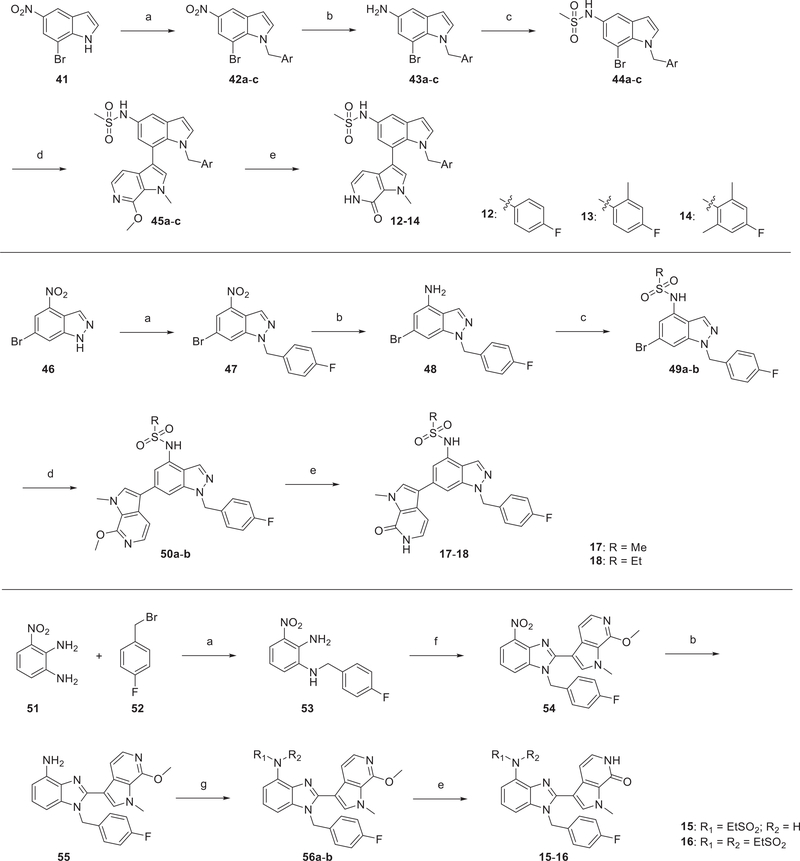
Scheme 2. Synthetic Route of Compounds 12–18a.
aReagents and conditions: (a) aryl bromide, K2CO3, DMF, RT, 2 h, 75–96%; (b) Fe, saturated NH4Cl (aq), EtOH, reflux, 2 h, 66–92%; (c) sulfonyl chloride, pyridine, RT, 1–5 h, 83–90%; (d) 35, Pd(dppf)Cl2–DCM, K3PO4, dioxane/H2O, 6–24 h, 90 °C, 75–95%; (e) TMSCl, NaI, MeCN/H2O, 2 h, 50 °C, 87–95%; (f) 36, AcOH, NaBO3, 50 °C, 1 h, 67%; (g) ethanesulfonyl chloride, triethylamine, DCM, RT, 3 h, 83–90%.