Fig 3.
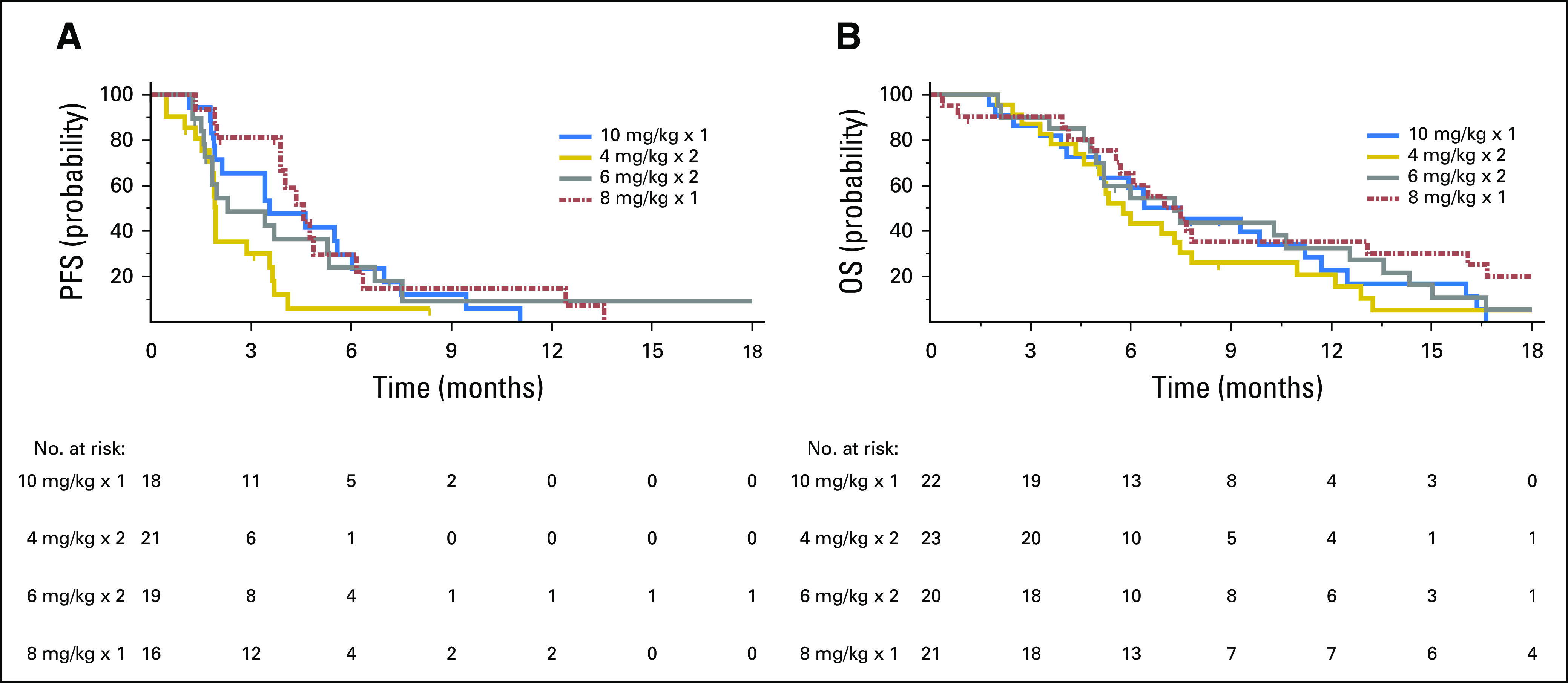
(A) Progression-free survival (PFS) and (B) overall survival (OS) in patients with refractory metastatic colorectal cancer treated with labetuzumab govitecan once weekly at 8 or 10 mg/kg or twice weekly at 4 or 6 mg/kg. Of the 86 patients, 72 continued on-study until progressive disease was documented radiologically at a tumor response assessment, whereas the other 21 patients discontinued study participation before radiologic confirmation of progression and were censored for PFS at the time of their most recent radiologic evaluation. Similarly, 78 of the 86 patients were observed until death, whereas eight patents were lost to follow-up and were censored for OS at last study evaluation.
