FIGURE 1.
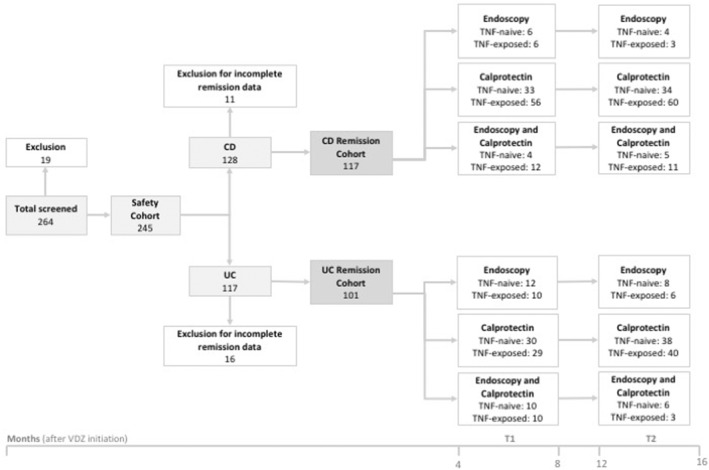
Flow chart describing the number of patients included in the study. In the safety and discontinuation subcohort (N = 245), AEs and reasons for discontinuation of VDZ treatment were investigated. In the remission subcohort, calprotectin and endoscopic remission rates were determined in two time frames after VDZ initiation: Months 4–8 (T1) and Months 12–16 (T2). AEs, adverse events; CD, Crohn's disease; UC, ulcerative colitis; VDZ, vedolizumab
