Figure 1 -.
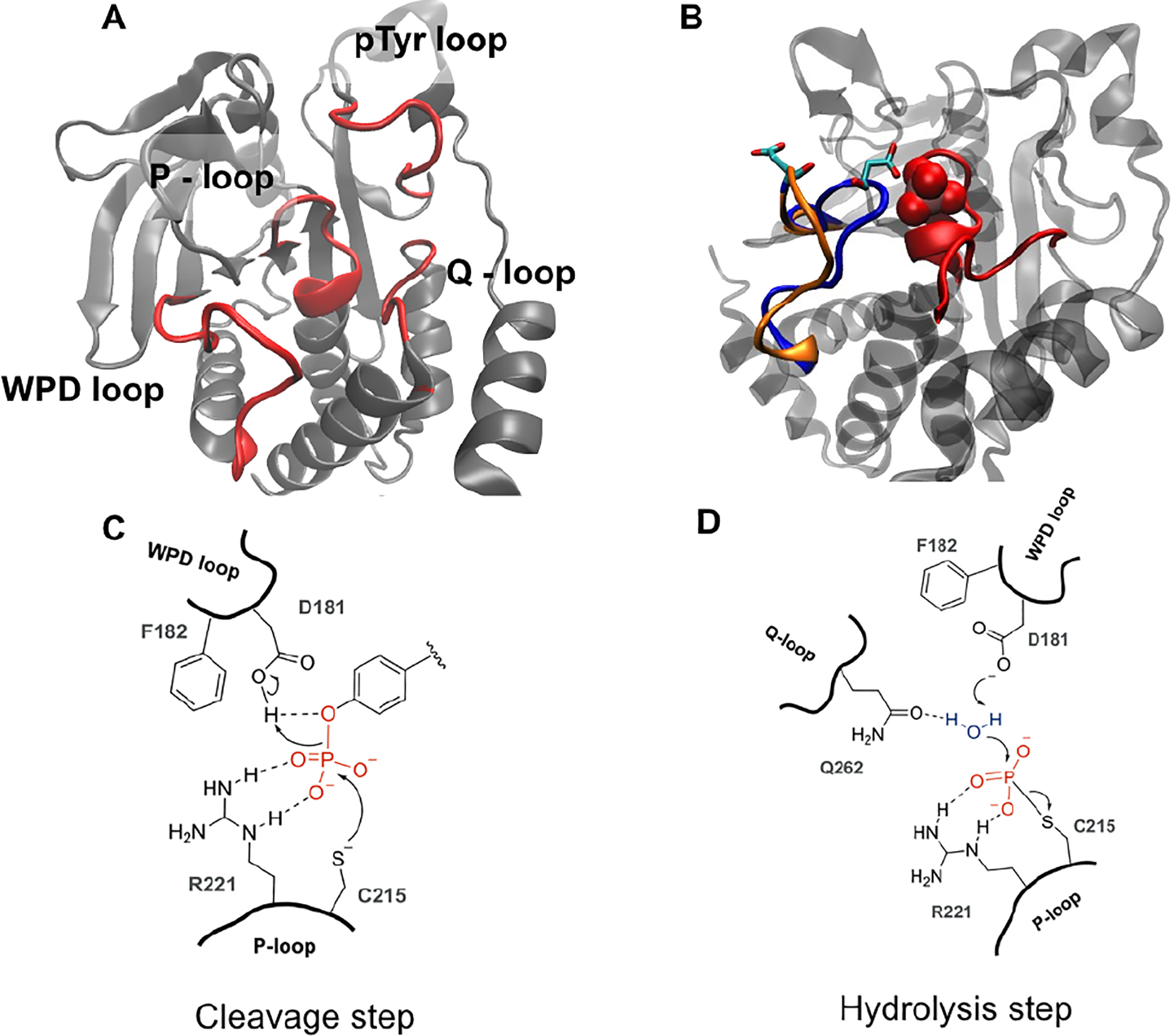
A) Cartoon rendering of PTP1B (PDB: 5k9v) with active site loops in red B) WPD loop in the open (orange) and closed (blue) conformation. The catalytic acid, D181 is shown in stick representation. Upon substrate (or vanadate, shown in red space-filling in B) binding, the WPD loop closes over the active site. C) In the cleavage step, C215 acts as a nucleophile while D181 donates a proton in the cleavage of the O-P bond of the phosphotyrosine to generate a phosphocysteine intermediate. D) D181 and Q262 enable a water molecule in the hydrolysis step to regenerate the apo enzyme.
