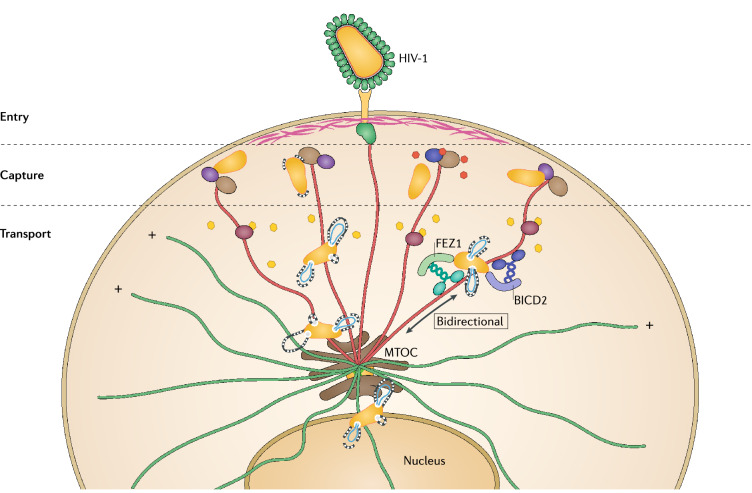
Fig. 1.
HIV-1 interactions with the host cell cytoskeleton during early infection. Entry. Upon entry by fusion with specific receptors at the plasma membrane, incoming HIV-1 cores penetrate through cortical actin (pink) and are deposited in the cytoplasm. Soon after entry, HIV-1 induces microtubule (MT) stabilization (shown as red filaments), which is regulated in part by actin-MT cross-linking proteins (green). Capture. Fusion of HIV-1 into the cytosol releases matrix (MA) protein (orange) that is captured by a complex of MT plus-end tracking proteins (+TIPs) consisting of the MT end-binding protein (EB1) (light brown) and Kif4 (dark blue) at the tip of MTs (+) to induce MT stabilization. The incoming conical capsid (yellow) is also captured with the EB1-associated actin-MT crosslinkers Dia1/2 (purple) to induce additional MT stabilization. The SxIP containing EB1-associated +TIPs CLIP170 and CLASP2 (dashed lines around the capsid) bind to and stabilize capsid, perhaps via recognition of local capsid lattice ruptures induced by the onset of reverse transcription, to upload the incoming cores from cortical actin onto stable MTs. Transport. The capsid binds MT motor adaptors BICD2 and FEZ1 to bridge viral particles to dynein (dark blue) and kinesin-1 (green) motors, respectively, to mediate their long-range bidirectional transport on stable MTs towards the nucleus. As the conical capsid moving along stable MTs loses small patches of CA to accommodate the outgrowing reverse transcribing viral genome (blue), CA protein release likely further increases MT stabilization over time via interactions with MT associated proteins MAP1A/S (dark red). Incoming cores accumulate at the perinuclear MT organizing center (MTOC) prior to nuclear entry by an as-yet unknown transport mechanism