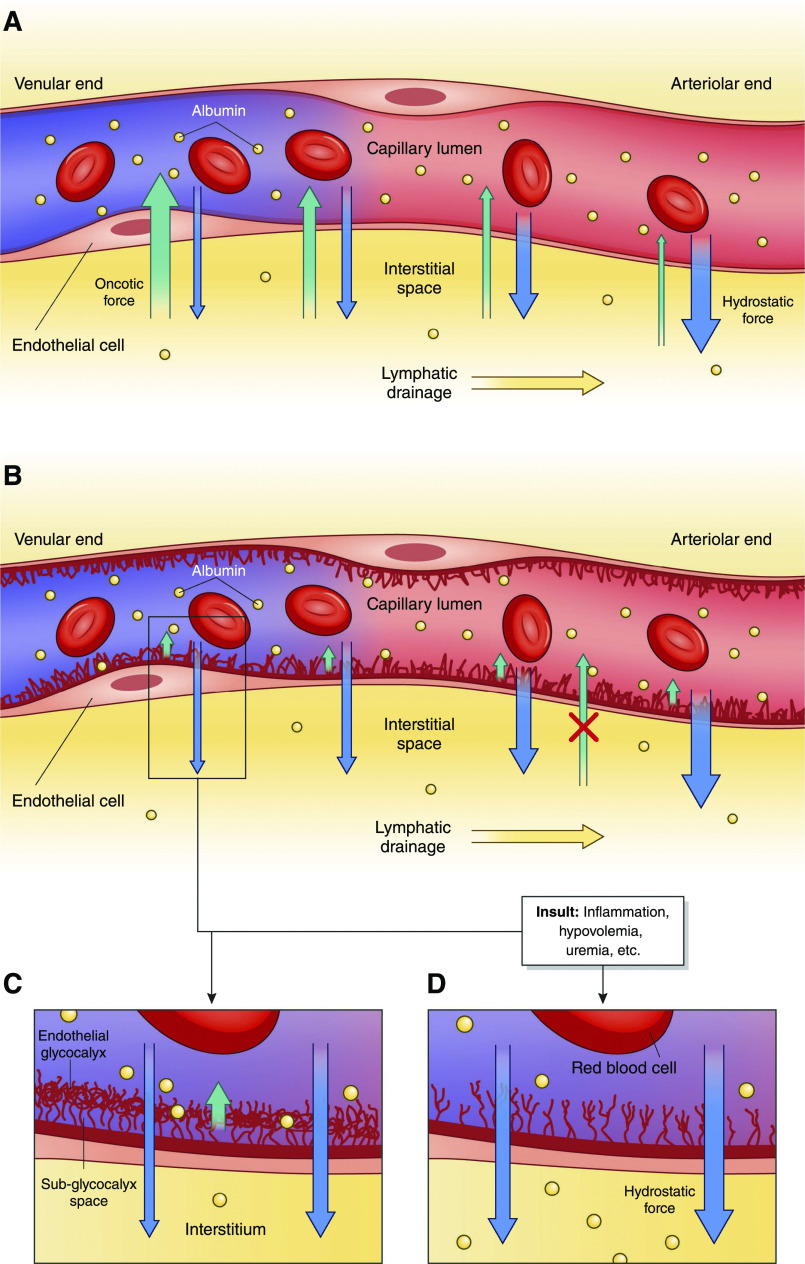
Figure 2.
Albumin movement across the capillary endothelium in health and disease. (A) In the historical model of capillary fluid exchange on the basis of Starlings forces, net plasma filtration was believed to occur at the arteriolar end of the capillary due to predominant hydrostatic force (blue arrows), which then transitioned to net reabsorption as plasma reached the venular end due to increasing oncotic drive from the interstitium, back into the capillary lumen (green arrows) (additional details are in Supplemental Material). Some research now suggests that there is, in fact, little to no reabsorption at the level of the capillary in the steady state (but it still may occur during acute hypovolemia). (B and C) A simplified version of the endothelial glycocalyx in its role as a selectively permeable barrier for fluid resorption. Hydrostatic pressure is the predominant force in this new model regulated by the glycocalyx, with little to no effect from osmotic forces between the vascular lumen and the interstitium. Instead, the endothelial glycocalyx functions to create an osmotic gradient (orange arrow) between the capillary lumen and the subglycocalyx space that opposes outward shift of fluid but does not reverse the direction of fluid flow. Both the interstitium and the subglycocalyx space have low oncotic content, resulting in negligible oncotic forces between the two spaces and overall net ultrafiltration throughout plasma transit in capillary microcirculation. Fluid that has shifted into the interstitium is resorbed solely through the lymphatic system, with exceptions (including the kidneys). (D) Fluid shift in the setting of a damaged endothelial glycocalyx from stressors, including inflammation, uremia, arteriosclerosis, hyperglycemia, and hypovolemia, among many others. With a damaged endothelial glycocalyx, capillaries have increased permeability to both serum proteins and fluid, leading to increased net fluid movement into the interstitium and interstitial edema when the lymphatic drainage capacity is exceeded.