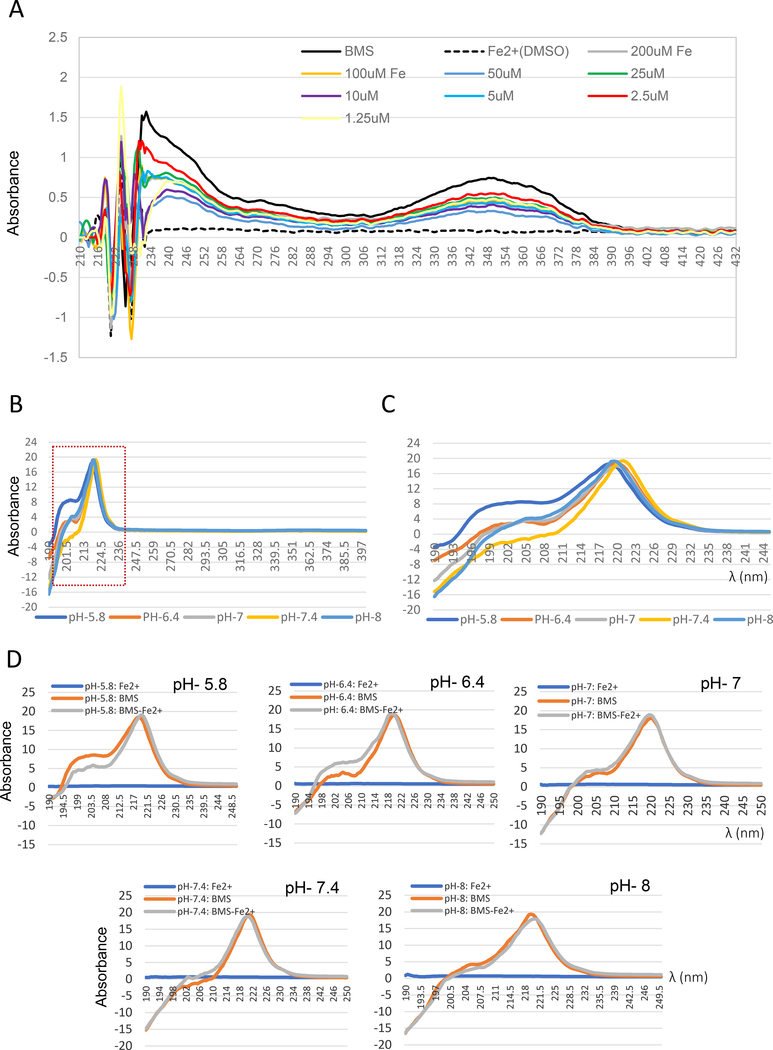
Figure 7. Evidence indicating an interaction of BMS536924 with iron.
(A) UV-Visible absorption spectrum of BMS536924 (100 μM) dissolved in water in the presence of indicated concentrations of iron (2+) in the form of ferrous sulfate. Absorbance from 190 nm to 430 nm is shown. (B-D) Effect of pH on BMS536924/Iron interaction. To test the effect of pH, BMS536924 was dissolved in 0.1M potassium phosphate buffer at pH 5.8, 6.4, 7, 7.4 or 8. (B) UV-Visible absorption spectrum of BMS536924 (100 μM) alone. Potassium phosphate buffer suppressed absorbance at 350 nm whereas absorbance at 220 nm persisted. (C) Effect of pH on BMS536924 absorbance from 190nm to 250nm. (D) Effect of iron on BMS536924 fluorescence at different pH. BMS536924 (100 μM) was mixed with 100 μM ferrous sulfate (iron 2+) and absorbance from 190 nm to 250 nm is shown.