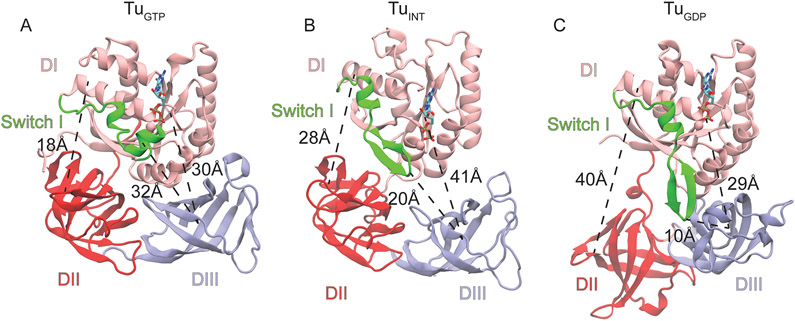
Figure 4. Conformations of EF-Tu during aa-tRNA accommodation.
(A) The classical closed conformation of EF-Tu bound to GDPNP (PDB ID: 1EFT) with a DI-DII distance of 18Å, DI-DIII distance of 30Å, and a switch I-DIII distance of 32Å. (B) The intermediate conformation of EF-Tu resolved by structure based simulations where the DI-DII and DI-DIII distances have increased to 28 and 41 Å, respectively and switch one is in an intermediate proximity to DIII at a distance of 20Å. (C) The classical open conformation of EF-Tu bound to GDP (PDB 1EFC). DI-DII is fully open at a distance of 40Å, DI has approached DIII and switch I is packing with DIII.