Abstract
About 95% of the ultraviolet (UV) photons reaching the Earth’s surface are UV-A (315–400 nm) photons. Plant responses to UV-A radiation have been less frequently studied than those to UV-B (280–315 nm) radiation. Most previous studies on UV-A radiation have used an unrealistic balance between UV-A, UV-B, and photosynthetically active radiation (PAR). Consequently, results from these studies are difficult to interpret from an ecological perspective, leaving an important gap in our understanding of the perception of solar UV radiation by plants. Previously, it was assumed UV-A/blue photoreceptors, cryptochromes and phototropins mediated photomorphogenic responses to UV-A radiation and “UV-B photoreceptor” UV RESISTANCE LOCUS 8 (UVR8) to UV-B radiation. However, our understanding of how UV-A radiation is perceived by plants has recently improved. Experiments using a realistic balance between UV-B, UV-A, and PAR have demonstrated that UVR8 can play a major role in the perception of both UV-B and short-wavelength UV-A (UV-Asw, 315 to ∼350 nm) radiation. These experiments also showed that UVR8 and cryptochromes jointly regulate gene expression through interactions that alter the relative sensitivity to UV-B, UV-A, and blue wavelengths. Negative feedback loops on the action of these photoreceptors can arise from gene expression, signaling crosstalk, and absorption of UV photons by phenolic metabolites. These interactions explain why exposure to blue light modulates photomorphogenic responses to UV-B and UV-Asw radiation. Future studies will need to distinguish between short and long wavelengths of UV-A radiation and to consider UVR8’s role as a UV-B/UV-Asw photoreceptor in sunlight.
In sunlight, UVR8 mediates the perception of both UV-B and short-wavelength UV-A radiation with its sensitivity moderated by blue light perceived through cryptochromes.
Introduction
Ultraviolet (UV) radiation (100–400 nm) is divided based on wavelength into UV-C (100–280 nm), UV-B (280–315 nm), and UV-A (315–400 nm) bands. These definitions originate from discussions held in 1932 and were later used for CIE and ISO standards (Björn, 2015). Wavelength limits were likely chosen based on the properties of DNA and ozone, and available instrumentation, without consideration of plant responses. Despite this, these limits have been used with only small variations almost unquestioned in plant research for nearly a century. This is in stark contrast to the definition of photosynthetically active radiation (PAR, 400–700 nm) that is based on measured action spectra (McCree, 1972).
In sunlight, the photon ratio between UV radiation and PAR is close to 0.1. Extraterrestrial UV-C and UV-B radiation of wavelength < 290 nm is absorbed in the atmosphere and more than 95% of the UV photon irradiance reaching the Earth’s surface falls within the UV-A region. The UV-A:PAR ratio is less affected by the length of the path through the atmosphere than the UV-B:PAR ratio and therefore it varies much less with sun elevation (Figure 1). Both ratios are, in turn, less affected by clouds than PAR irradiance itself (Figure 1).
Figure 1.
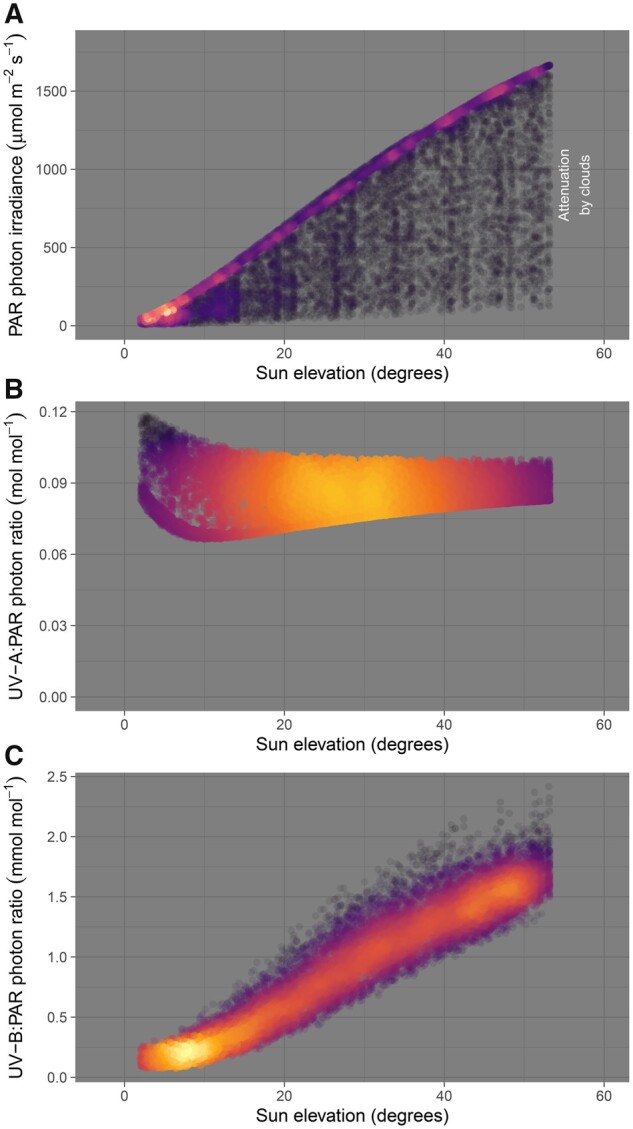
Sunlight during five summers at Kumpula, Helsinki (60.20400 N, 24.95811 E). Summaries computed from simulated hourly spectral solar irradiance at ground level are plotted against solar elevation above the horizon. A, Photon irradiance of PAR; B, UV-A:PAR photon ratio; C, UV-B:PAR photon ratio. The color indicates the local density of observations, with the “hotter” red to yellow regions mostly corresponding to data for clear-sky conditions and the “cooler” dark points corresponding to different degrees of cloud cover. Original data consist in 11,759 hourly simulations for sun elevation angles higher than 3–7 degrees at the center of the hour, for the period 1 May to 30 September of years 2013–2017, produced by Anders V. Lindfors with a radiation transfer model (libradtran) (Lindfors et al., 2009; Emde et al., 2016).
ADVANCES
The “UV-B” photoreceptor UVR8 mediates perception of both UV-A and UV-B radiation in sunlight.
Short and long wavelengths within the UV-A waveband are perceived through UVR8 and CRYs, respectively.
CRYs-dependent signaling drastically downregulates UVR8-mediated responses to UV-B and short-wavelength UV-A radiation.
Redundancy in photoreceptor function ensures tolerance of exposure to solar UV radiation, allowing survival of uvr8 and cry1cry2 mutants.
Multiple negative feedback loops downstream of UVR8 and CRYs moderate the responses they mediate and make possible the convergence of these responses towards steady states.
The detection of thinning of the ozone layer due to human activity nearly 40 years ago triggered strong interest in UV-B radiation as a possible stressor for plants. However, the current view is that solar UV radiation acts mainly as a regulator of growth and development of plants and only exceptionally as a stressor (Jenkins, 2017; Verdaguer et al., 2017). In UV-acclimated plants, the regulatory effect of UV radiation is predominantly mediated by photoreceptors or light-sensing pigments, while damage is avoided or readily repaired (Robson et al., 2019). However, with high UV-B doses, DNA damage itself can trigger regulatory responses (Dotto and Casati, 2017).
UV RESISTANCE LOCUS 8 (UVR8) is commonly described as a UV-B photoreceptor (Rizzini et al., 2011) while cryptochromes 1 and 2 (cry1, cry2, CRYs when referring to both), phototropins 1 and 2 (phot1, phot2, PHOTs), and three zeitlupe proteins are described as UV-A/blue photoreceptors (Briggs and Huala, 1999; Christie et al., 2015). These roles have been attributed to UVR8, CRYs, and PHOTs based on their strong photon absorption in these regions (Briggs and Christie, 2002; Banerjee et al., 2007; Christie et al., 2012), and on the responses to monochromatic radiation that they mediate (Rizzini et al., 2011; Christie et al., 2015; Jenkins, 2017; Robson et al., 2019). However, information on the role of photoreceptors in plant responses to UV-A radiation has been scarce. Most past studies on the function of CRYs and PHOTs have focused only on blue-light-induced responses, likely because of an expectation that the mechanism depends on the photoreceptor rather than on the wavelength. Similarly, most studies on the function of UVR8 have focused on UV-B. In addition, the role of cryptochrome 3 or cry-dash in perception and signaling of UV-A radiation and blue light remains unclear (Chaves et al., 2011). This had left a gap in our knowledge of photoreceptor-dependent responses to UV radiation. This gap was most notable for photoreceptor function in sunlight and shade light, as the artificial lighting used in most controlled-environment experiments has been very different in its spectrum and irradiance from those in the natural environment.
In this article, we review recent advances in our understanding of the role of photoreceptors in plant responses to solar UV radiation. We discuss how the action of photoreceptors depends on the shape of the solar spectrum and how responses are dependent on the joint action of photoreceptors. With future research in mind, we highlight the current challenges faced by research on plants’ responses to solar UV radiation and suggest ways of addressing them. Whole-plant responses to UV-B and UV-A radiation (Jenkins, 2017; Verdaguer et al., 2017; Jansen and Urban, 2019), responses not mediated by photoreceptors (Hideg et al., 2013; Jenkins, 2017), as well as details of the perception of UV-B radiation (Jenkins, 2017; Yin and Ulm, 2017), already covered by recent reviews are beyond the scope of this update.
Photoreceptors and responses
The role of CRYs, PHOTs, and zeitlupe proteins in the regulation of multiple plant responses to blue light has been well demonstrated and reviewed (Briggs and Christie, 2002; Chen et al., 2004; Yu et al., 2010; Christie et al., 2015; Yang et al., 2017). CRYs mediate most blue-light-induced changes in gene expression, as well as cotyledon expansion, accumulation of phenolic metabolites, and regulation of flowering time (Kleine et al., 2007; Yu et al., 2010; Christie et al., 2015; Wang et al., 2017). PHOTs mediate phototropism and chloroplast movement (Briggs and Christie, 2002; Christie et al., 2015), whereas both CRYs and PHOTs mediate hypocotyl-growth inhibition and stomatal opening under blue light (Folta and Spalding, 2001; Wang et al., 2020).
Similarly, the role of UVR8 in the perception of UV-B radiation has been clearly demonstrated and reviewed (Tilbrook et al., 2013; Jenkins, 2014; Jenkins, 2017; Yin and Ulm, 2017). UVR8 mediates UV-B-induced changes in gene expression and photomorphogenic responses such as inhibition of hypocotyl elongation, promotion of cotyledon expansion, induction of flavonoid biosynthesis, and accumulation of flavonoid compounds (Brown et al., 2005; Favory et al., 2009; Demkura and Ballaré, 2012; Morales et al., 2013; Jenkins, 2017; Yin and Ulm, 2017; Rai et al., 2019). UVR8 is required for UV-B-induced phototropism of the Arabidopsis (Arabidopsis thaliana) inflorescence (Vanhaelewyn et al., 2019), the down-regulation of growth-related genes by UV-B radiation (Mazza and Ballaré, 2015), and the repression of shade avoidance by sun flecks (Moriconi et al., 2018). UVR8 also regulates UV-B-induced stomatal closure in Arabidopsis (Tossi et al., 2014). In etiolated Arabidopsis seedlings, only in the absence of PHOTs, UVR8 participates in UV-B-induced phototropism, whereas PHOTs regulate this response if present (Vandenbussche et al., 2014). Additional evidence for a role of phot1 in responses to UV-B radiation comes from a study on chloroplast movement in response to UV-B in detached Arabidopsis leaves (Hermanowicz et al., 2019). However, the mechanism by which PHOTs participate in UV-B responses is still unknown.
In comparison to UV-B radiation and blue light, fewer studies have addressed the role of plant photoreceptors in the perception of UV-A radiation (Lin et al., 1995; Liscum and Briggs, 1995; Fuglevand et al., 1996; Lin et al., 1996). Based on few studies done in controlled environments using “blacklight blue” lamps with peak of emission at 350 or 368 nm, it is known that cry1 mediates suppression of hypocotyl elongation, accumulation of anthocyanins, and induction of the flavonoid biosynthesis gene CHALCONE SYNTHASE (CHS) (Lin et al., 1995; Fuglevand et al., 1996; Lin et al., 1996), while phot1 mediates phototropism (Liscum et al., 2003). An outdoor experiment assessing responses to solar UV-A provided the first evidence for the possible involvement of UVR8 in UV-A-mediated changes in gene expression and accumulation of specific phenolic compounds (Morales et al., 2013). It was proposed that under solar PAR and UV-A irradiance, UVR8 interacted with other photoreceptors through signaling pathways to modulate UV-A responses in the presence of UV-B radiation (Morales et al., 2013).
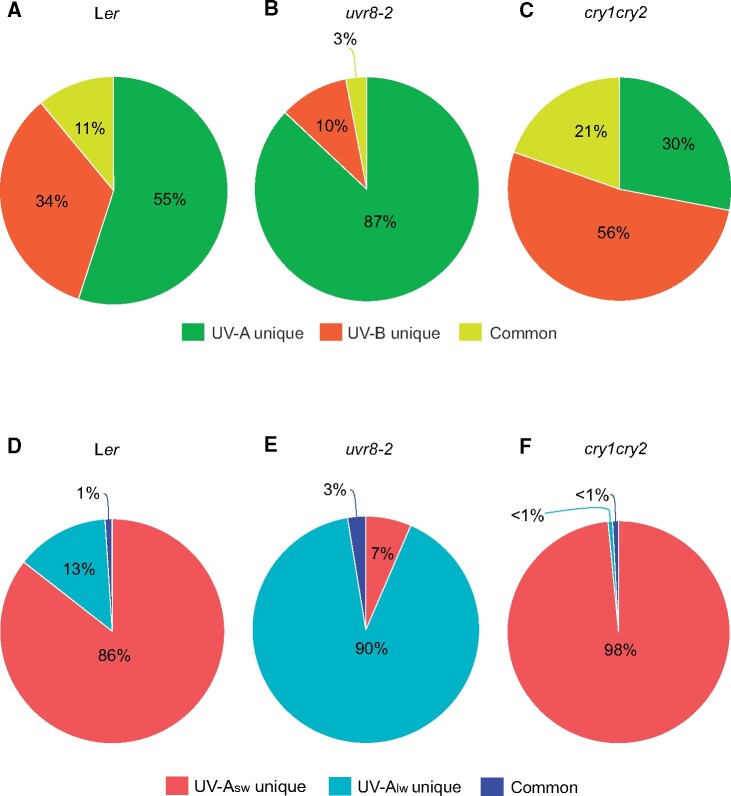
In a recent study, using photoreceptor mutants in sunlight, Rai et al. (2020) showed that both UVR8 and CRYs mediate transcriptome-wide responses to solar UV-A (315–400 nm; Figure 2, A–C). However, within UV-A, the roles of UVR8 and CRYs differed: UVR8 was required for the responses to short-wave UV-A radiation (315–350 nm, UV-Asw; Figure 2, D and E), while CRYs were required for responses to long-wave UV-A radiation (350–400 nm, UV-Alw; Figure 2, D and F). This split at approximately 350 nm is also consistent with earlier studies reporting UVR8-independent induction of CHS in response to UV-A wavelengths between 350 and 400 nm (Brown et al., 2005) and UVR8-independent inhibition of stomatal opening by UV-A at 380 nm (Isner et al., 2019). Given these results, the relevance of the definition of the UV-A waveband (315–400 nm) to plants needs to be re-assessed (Box 1).
Figure 2.
Transcript abundance after 6 h exposure to filtered sunlight in three genotypes of Arabidopsis plants. A separate pie chart for each genotype shows the percentage of differentially expressed genes responding uniquely to UV-B radiation (orange), uniquely to UV-A radiation (green), and common to both UV-B and UV-A radiation (yellow). A–C, responding uniquely to short-wavelength UV-A (UV-Asw) radiation (red), uniquely to long-wavelength UV-A (UV-Alw) radiation (light blue), and common to both UV-Asw and UV-Alw radiation (dark blue). D–F, Figure based on the transcriptome analysis of Rai et al. (2020).
BOX 1.
Photoreceptor action and the definition of the UV-A waveband
Rai et al. (2020) showed that solar UV-Asw (315–350 nm) was far more effective than solar UV-Alw (350–400 nm) in the regulation of transcript abundance across the whole transcriptome. Effectiveness was assessed based on the number of genes and the magnitude of response for selected individual genes, both of which were many times higher in UV-Asw than UV-Alw. The transcriptomic analysis also showed that different sets of genes were triggered by UV-Asw and UV-Alw in the WT, with only 1% of genes in common (Figure 2D), while the overlap was larger when considering UV-B and whole of UV-A (Figure 2A). These results indicated that in sunlight the main role of CRYs is in responses to blue light, whereas in responses to UV-A radiation their role is minor compared to that of UVR8 (Rai et al., 2020). As monomerization of UVR8 dimers driven by photon absorption is considered crucial for its action (Rizzini et al., 2011), that the purified UVR8 protein monomerizes in vitro only upon exposure to wavelengths of 335 nm or shorter, helps explain why UVR8’s action is limited to the UV-B and UV-Asw regions (Rai et al. 2020). UVR8 is an unusual plant photoreceptor where tryptophan amino acids within the UVR8 protein behave as chromophore (Christie et al., 2012), and in that excitation transfer among the three tryptophan groups, which absorb maximally at slightly different wavelengths results in enhanced quantum efficiency (Li et al., 2020). This photochemical mechanism has been considered capable of explaining the transition in UVR8 function at a wavelength near 350 nm (Li et al., 2020). Furthermore, it is possible that this cut-off wavelength observed in vitro is slightly different in vivo. Thus, the UV-A waveband can be divided into two different regions (UV-Asw and UV-Alw) based on a transition at 335 nm to 350 nm between radiation-dependent action of UVR8 and CRYs in the regulation of gene expression (Rai et al., 2020). Based on the mechanism behind the transition, the wavelength boundary observed in Arabidopsis can be expected to be similar to that in other plant species, although further studies are needed to confirm this.
CRYs may not have a direct role in responses to UV-B and UV-Asw radiation, but when activated by UV-Alw and PAR, CRYs negatively regulate the UVR8-mediated gene expression occurring in response to UV-B and UV-Asw (Rai et al., 2019, 2020; Tissot and Ulm, 2020). The double mutant cry1cry2 had a stronger gene-expression response to UV-B and UV-Asw radiation than the WT (Figure 2, D and F; Rai et al., 2019, 2020; Tissot and Ulm, 2020). Furthermore, Tissot and Ulm (2020) dissected mechanistically the regulation of UV-B responses by CRYs with an involvement of REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2, the negative feedback regulator of UVR8 signaling (see the “Molecular mechanisms of photoreceptor action” section). Thus, although UVR8 might be the primary sensor of UV-B and UV-Asw radiation, CRYs-mediated blue light signaling negatively regulates the activity of the UVR8 photoreceptor (Rai et al., 2019, 2020; Tissot and Ulm, 2020). This highlights the need for direct evidence supporting photoreceptor activation at specific wavelengths, as altered responses in photoreceptor mutants provide only circumstantial evidence due to regulatory interactions.
As growth and survival are important determinants of plants’ fitness in nature, it is relevant to understand how photoreceptors regulate these responses both in the UV-B and UV-A regions. It has been shown that UVR8, CRYs, and a red/far-red photoreceptor phytochrome B (phyB) modulate plant growth in response to UV-B, UV-Asw, and UV-Alw, in the presence of PAR (Rai et al., 2019; Tissot and Ulm, 2020). If either of UVR8 or CRYs are present, plants grew normally in response to UV-B, UV-Asw, and UV-Alw radiation; however, when UVR8 and CRYs were simultaneously absent in mutants, growth was drastically reduced (Rai et al., 2019; Tissot and Ulm, 2020). Furthermore, CRYs and phyB act redundantly with UVR8 to provide UV-B tolerance in plants (Rai et al., 2019; Tissot and Ulm, 2020). This indicates that UVR8 and CRYs or UVR8 and phyB can substitute for each other in triggering acclimation to sunlight allowing Arabidopsis plants to grow normally. However, under harsher environmental conditions substitution between UVR8 and CRYs or UVR8 and phyB could be less effective in maintaining plants’ fitness.
Optical phenomena
The likelihood of photons being absorbed by a photoreceptor depends both on the absorption properties of the photoreceptor and on the wavelength and number of photons impinging on it. Sensing also requires that the excitation of the photoreceptor is transduced into a downstream response. In cotyledons of Arabidopsis seedlings, expression of UVR8 in the epidermis and in the mesophyll in a UVR8 null-mutant background using tissue-specific promoters contributed to regulation of the expression of ELONGATED HYPOCOTYL 5 (HY5) and EARLY LIGHT-INDUCED PROTEIN 2 (ELIP2) locally within each of these tissues (Bernula et al., 2017). In the control, using the native promoter, UVR8 was expressed in the epidermis more than in the mesophyll, while not detected in the vascular tissue (Bernula et al., 2017). In leaf samples collected from plants of 42 species growing outdoors, species’ mean epidermal transmittance to UV-B radiation (300 nm) varied between less than 1% and 58% (Day et al., 1993). The spectrum and direction of the radiation incident on leaf surfaces is altered by absorption and reflection before reaching plant tissues where the photoreceptors are located (Day et al., 1993; Brodersen and Vogelmann, 2010).
Phenolic metabolites accumulated on the cuticle and in epidermal cells strongly absorb UV radiation (Krauss et al., 1997; Siipola et al., 2015) while cuticular waxes usually reflect both UV radiation and PAR (Holmes and Keiller, 2002). In plants, the accumulation of phenolic metabolites is regulated by radiation perceived through CRYs, UVR8, and phytochromes and is responsive to UV-B, UV-A, blue, red, and far-red wavelengths (Duell-Pfaff and Wellmann, 1982; Holopainen et al., 2018). In sunlight, their accumulation frequently depends predominantly on UV-B radiation (Morales et al., 2010, 2013) but occasionally predominantly on blue light (Siipola et al., 2015). Accumulation of phenolic metabolites in epidermis apparently depends to a large extent on direct exposure of each epidermis to UV radiation (Morales et al., 2011; Bidel et al., 2015; Solanki et al., 2019; Pieristè et al., 2020).
UV absorptance and reflectance determine the transmittance of the epidermis, which can adapt across generations as a result of natural selection, acclimate over several days and, in some species, even track changes in ambient solar irradiance within tens of minutes (Veit et al., 1996; Siipola et al., 2015; Barnes et al., 2016b, 2017). For example, in barley leaves, both epidermal UV-A and UV-B transmittances varied between 35% and 2% in plants subjected to different conditions including low PAR in the absence of UV-B radiation (Kolb and Pfündel, 2005). In the same species, mean epidermal UV-A transmittance decreased from 56% to 10% in the course of 1 week during acclimation to UV exposure (Klem et al., 2015). In a few species, epidermal UV-A transmittance can vary by 50% or more through the day while in other species the range of variation is much smaller (Barnes et al., 2016a, 2016b). Variation through the course of a day has been shown to require exposure to UV radiation shorter than 350 nm (Veit et al., 1996). Epidermal UV-A transmittance also varies seasonally (Pescheck and Bilger, 2019; Solanki et al., 2019). UVR8 is located both in the epidermis and mesophyll tissues where it participates in signaling, controlling the accumulation of the same phenolic metabolites that attenuate the UV radiation entering the leaf (Bidel et al., 2015). Consequently, conforming a signaling loop that could play an important role in stabilizing plant responses to UV radiation through negative feedback (Bidel et al., 2015). The screening by phenolics is much weaker in the blue waveband than in the UV waveband, and so also the gain of the negative feedback must be weaker, consequently more strongly affecting UVR8- than CRYs-dependent signaling. There is yet no direct evidence supporting this hypothesis, but it is grounded in physical principles.
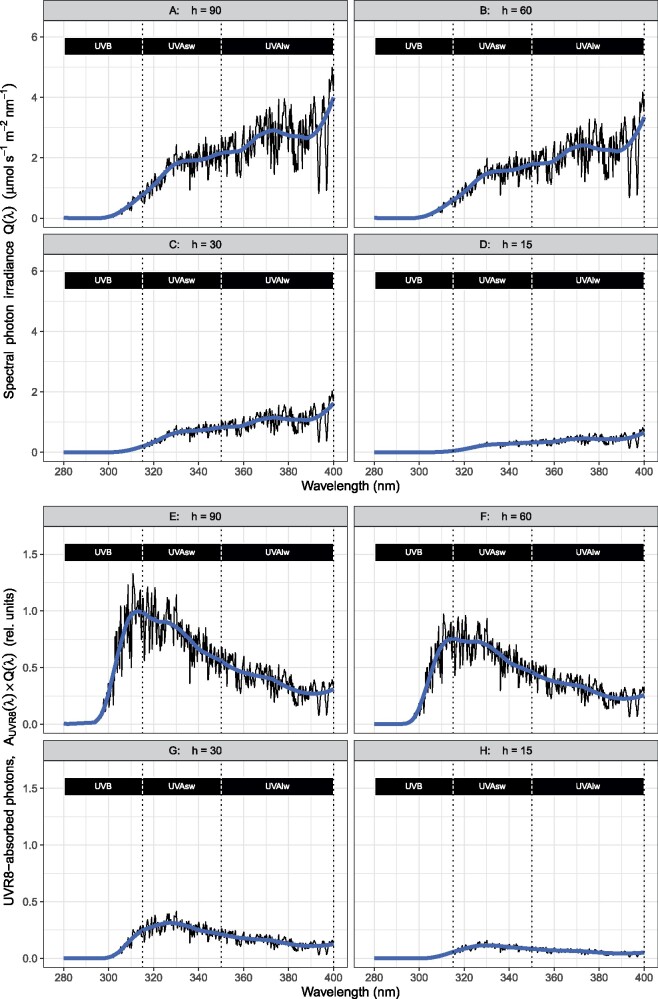
In addition to screening by pigments, the spectrum of radiation incident on a plant determines the activation of photoreceptors. To estimate the absorption of photons by a photoreceptor in planta, we would need to know the spectral photon irradiance incident on the photoreceptor molecules and the absorption spectrum of the photoreceptor in planta. However, both quantities are difficult or impossible to measure with current methods. Notwithstanding these limitations, combining the in vitro absorption spectrum of a photoreceptor with the spectrum of radiation incident on a plant provides a crude estimate of the relative number of photons a photoreceptor could absorb at different wavelengths, as long as these wavelengths are similarly attenuated in the epidermis. By combining the in vitro absorption spectrum of the UVR8 protein, measured over the whole UV-B and UV-A regions with hourly spectral irradiances, Rai et al. (2020) showed that the steep increase in solar spectral irradiance near the boundary between UV-A and UV-B regions is enough to allow UVR8 to abundantly absorb UV-A photons, thus explaining the observed role of UVR8 in solar UV-A responses (Morales et al., 2013; Rai et al., 2020). In Figure 3, using spectral irradiance for different sun elevations above the horizon, we additionally show that UVR8 is likely to mediate the perception of UV-Asw radiation both when the sun is at the zenith and the UV-B:UV-A photon ratio is at its maximum and when it is lower in the sky. This suggests that UV-Asw photons perceived through UVR8 could contribute to responses to solar radiation throughout the photoperiod and at low and middle latitudes, throughout the year, that is even when solar UV-B irradiance is very weak. However, downregulation of UVR8 action by blue light absorbed by CRYs would moderate downstream responses.
Figure 3.
Solar UV radiation at different solar elevations and corresponding estimates of absorbed photons by photoreceptor UV RESISTANCE LOCUS 8 (UVR8) molecules. Panels (A)–(D) show modeled solar spectrum for clear sky conditions and sun elevation angles (h) of 90, 60, 30, and 15 degrees. Panels (E)–(H) show result from convolution of the spectra in (A)–(D) with the in vitro absorbance spectrum of the UVR8 protein (Rai et al., 2020, Supplemental Figure S7) predicting that UVR8 will absorb both UV-B and UV-A radiation in sunlight. The solar spectrum was simulated with the Quick TUV simulator for a depth of the ozone layer of 300 DU. Computations and plotting were done in R (R Core Team, 2020) with packages from the R for photobiology suite (Aphalo, 2015) and the tidyverse (Wickham et al., 2019). UV-Asw: short wavelength UV-A, UV-Alw: long wavelength UV-A.
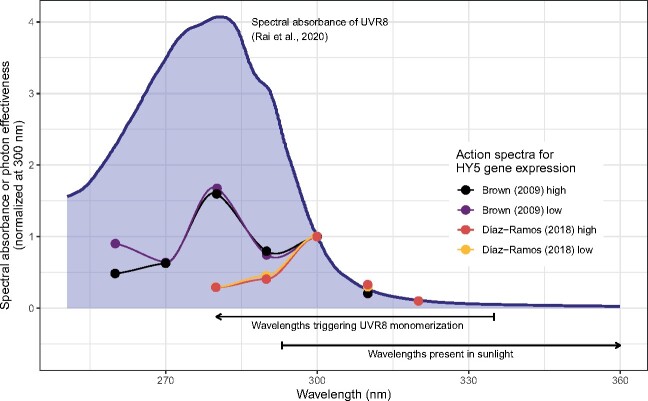
The theoretical expectation is that in the absence of differential screening by other pigments, an action spectrum will have a shape similar to that of the absorption spectrum of the photoreceptor (Gorton, 2010). Currently available UVR8 action spectra (Brown et al., 2009; Díaz-Ramos et al., 2018), both for HY5 expression at wavelengths 260–310/320 nm, can be scaled to match each other at wavelengths between 300 and 315 nm, and when this is done, their shape in this region resembles that of the absorption spectrum (Figure 4). However, at shorter wavelengths, the mismatch is large. Differences in the optical properties of leaves or damage caused by some wavelengths could explain the unexpectedly weak expression of HY5 at wavelengths shorter than 290 nm, wavelengths that are anyway absent from sunlight. As the seedlings used were grown under very low light (Brown et al., 2009; Díaz-Ramos et al., 2018), a condition where accumulation of flavonoids and other UV-screening pigments is reduced (Klem et al., 2015), these action spectra could differ from those for plants growing in sunlight. As different flavonoids and phenolic acids differ in the wavelengths of maximal absorption (Kolb et al., 2005), changes in phenolic composition could also alter the shape of the action spectrum. An equivalent effect of screening by chlorophyll on the action spectra of phytochromes is well documented (Gorton, 2010) and, as discussed above, epidermal absorption in the UV region can vary widely, suggesting a similar effect on UVR8 action spectra.
Figure 4.
Comparison of published action spectra for UV RESISTANCE LOCUS 8 (UVR8)-mediated expression of the gene ELONGATED HYPOCOTYL 5 (HY5) (Brown et al., 2009; Díaz-Ramos et al., 2018) to the published absorption spectrum of UVR8 (Rai et al., 2020). High and low refer to action spectra computed for different levels of monomerization, as reported in the original publications. All spectra are normalized to one at 300 nm.
Due to the interactions involving UVR8 and CRYs signaling, UVR8 action spectra measured using monochromatic light are unlikely to describe the action of UVR8 in sunlight. Polychromatic action spectra (see Cooley et al., 2000b) measured using photoreceptor mutants as controls would help disentangle the roles of UVR8 and CRYs in the perception of solar UV-A and UV-B radiation. Such an action spectrum is not yet available.
Molecular mechanisms of photoreceptor action
The excitation mechanism of absorption of a photon by a photoreceptor is likely to be the same irrespective of wavelength. Therefore, signaling mechanisms initiated by photoreceptors have been frequently studied using a few wavelengths, for example UV-B centered at 310 to 315 nm for UVR8 and blue light centered at 430 or 460 nm for CRYs and PHOTs. These mechanisms will be discussed here in relation to their role in the perception of solar UV radiation as they have been recently reviewed from a molecular perspective (Binkert and Ulm, 2017; Hideg and Strid, 2017; Jenkins, 2017; Liscum et al., 2020; Wang and Lin, 2020).
In sunlight and shade light, multiple photoreceptors are activated simultaneously making interactions downstream of them important for whole-plant responses (Casal, 2013; Ballaré and Pierik, 2017). Changes in natural illumination are usually gradual, allowing negative feedback to work effectively. As UVR8 and CRYs signaling interact to regulate gene expression in response to UV-B and UV-A radiation and to blue light (Rai et al., 2019, 2020), responses to solar UV radiation also depend on visible light. Consequently, molecular and signaling interactions can play a crucial role in the mechanism of UV perception in plants’ natural environment.
In the absence of UV-B, UVR8 exists as a homo-dimer and is mainly present in the cytosol, whereas UV-B exposure leads to UVR8 monomerization and rapid accumulation in the nucleus (Brown et al., 2005; Kaiserli and Jenkins, 2007; Rizzini et al., 2011). More recently it was also reported that UVR8 can monomerize under wavelengths in the UV-A region (up to ∼335 nm, Rai et al., 2020) which could be explained by the transfer of excitation energy between the three groups of tryptophan amino acids in the UVR8 protein (Li et al., 2020, see Box 1). Conversion from a dimer to monomer upon exposure to UV-B (Rizzini et al., 2011), followed by mobilization of the monomers from the cytosol to the nucleus are key steps in UVR8 signaling (Kaiserli and Jenkins, 2007). Conversely, upon activation by blue light, CRYs monomers form dimers (Wang et al., 2016). Cry1 is present both in the nucleus and cytosol whereas cry2 is present mainly in the nucleus (Guo et al., 1999; Wu and Spalding, 2007; Yu et al., 2007). After UV-B exposure, UVR8 interacts with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), a repressor of photomorphogenesis in darkness (Favory et al., 2009; Podolec and Ulm, 2018; Lau et al., 2019). This binding into the UVR8–COP1 complex inhibits the repressor activity of COP1 E3 ubiquitin ligase and stabilizes the HY5 transcription factor (TF), a master regulator of gene expression (Favory et al., 2009; Huang et al., 2013; Gangappa and Botto, 2016; Podolec and Ulm, 2018). Similarly, CRYs also bind to COP1 and inhibits its E3 ubiquitin ligase activity which stabilizes HY5 (Hoecker, 2017; Holtkotte et al., 2017; Podolec and Ulm, 2018; Lau et al., 2019). As signaling downstream of UVR8 and CRYs has many components in common, multiple points of interaction can be envisaged.
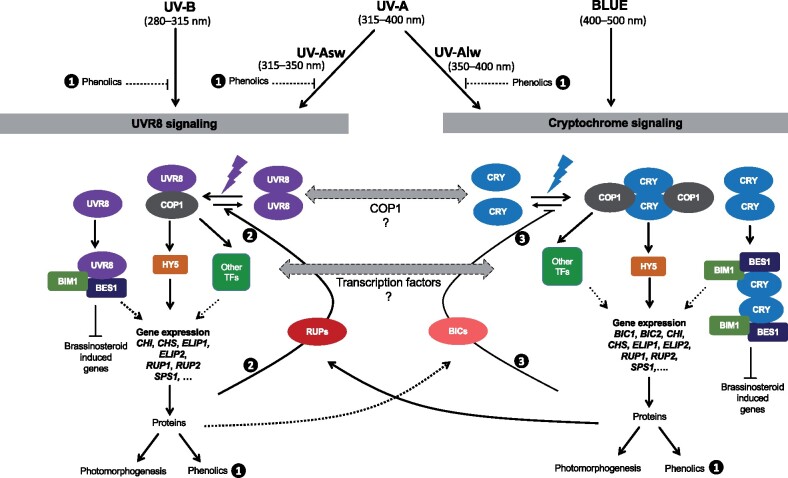
We hypothesize that the interactions downstream of UVR8 and CRYs could take place at multiple levels, as summarized in our model (Figure 5). The first level of interaction would depend on COP1, whose WD40 domain is the site for binding with the VP-peptide motif on UVR8 and CRYs (Lau et al., 2019; Ponnu et al., 2019). However, experimental evidence for competition between the photoreceptors for binding to COP1 is still lacking. Specific TFs could operate at the second level of interaction, as both UVR8 and CRYs signaling involves some of the same TFs for the regulation of gene expression, such as HY5, BRI1-EMS-SUPPRESSOR1 (BES1), BES1-INTERACTING MYC-LIKE 1 (BIM1), and PHYTOCHROME INTERACTING FACTORs (PIFs) (Hayes et al., 2014; Gangappa and Botto, 2016; Pedmale et al., 2016; Liang et al., 2018; Wang et al., 2018). The third level of interaction could involve RUP1 and RUP2, and BLUE-LIGHT INHIBITOR OF CRYPTOCHROMES 1 (BIC1) and BIC2; which are the negative feedback regulators of UVR8 photocycle and CRYs photocycle, respectively (Gruber et al., 2010; Heijde and Ulm, 2013; Findlay and Jenkins, 2016; Wang et al., 2017). RUPs act as negative regulators of UVR8 signaling by facilitating redimerization of UVR8 monomers (Heijde and Ulm, 2013; Findlay and Jenkins, 2016), while BICs act as negative regulators of CRYs signaling by inhibiting CRYs dimerization (Wang et al., 2017). CRYs signaling activated by blue light induces RUP1 and RUP2 gene expression and RUP2 protein accumulation, consequently enhancing UVR8 redimerization (Tissot and Ulm, 2020). Reciprocally, UVR8 signaling activated by UV-B radiation induces BIC1 and BIC2, and overexpression of BIC1 and BIC2 suppresses the CRYs-mediated UVR8 redimerization (Tissot and Ulm, 2020). Further downstream, other TFs could contribute to differential regulation of groups of genes (Rai et al., 2020). Effects of solar UV on plant-hormone signaling reflected in growth and development (Vanhaelewyn et al., 2016; Fina et al., 2017) can also give rise to interactions and feedback. Signaling can involve chained metabolic steps that by introducing delays contribute to whole-system dynamic properties (Chappell and Hahlbrock, 1984; Liao et al., 2020). In addition, we cannot yet rule out the possibility that physical interaction between the photoreceptors or other mechanisms of interaction is also involved.
Figure 5.
A model combining different hypotheses for coaction downstream of UV RESISTANCE LOCUS 8 (UVR8) and cryptochromes 1 and 2 (CRYs) in UV responses and possible modulation by other wavelengths. We postulate a first level of interaction through CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) as both UVR8 and CRYs physically interact with COP1, second level through shared TFs (e.g. ELONGATED HYPOCOTYL 5 [HY5], BRI1-EMS-SUPPRESSOR1 [BES1], BES1-INTERACTING MYC-LIKE 1 [BIM1], note that both UVR8 and CRYs physically interact with BES1 and BIM1*), and a third level through REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2, and BLUE-LIGHT INHIBITOR OF CRYPTOCHROMES 1 (BIC1) and BIC2. The complete arrows show paths supported by experimental evidence while the dotted arrows show hypothetical mechanisms that are compatible with current knowledge. Numbers 1–3 refer to the negative feedback loops described in Box 2. CHI: CHALCONE ISOMERASE, CHS: CHALCONE SYNTHASE, ELIP 1: EARLY LIGHT-INDUCED PROTEIN 1, ELIP2: EARLY LIGHT-INDUCED PROTEIN 2, SPS1: SOLANESYL DIPHOSPHATE SYNTHASE 1 (* not shown in the model).
The complexity of the signaling interactions downstream of UVR8 and CRYs suggests the need to study the perception of UV (and visible) radiation by plants as an integrated sensory system in which whole-system properties are largely determined by these interactions, that is whole-plant responses to sunlight cannot be predicted based solely on the responses to individual wavelengths or on the roles played by individual photoreceptors (Box 2).
BOX 2.
Negative feedback and redundancy
In the model in Figure 5, we have highlighted the negative feedback loops. In any control system, negative feedback contributes to stability. However, if a step increase in an input, here radiation, occurs faster than the response of the feedback loop, the response will overshoot before stabilizing. The phenolic synthesis loop, labelled 1 in Figure 5, contributes to long-term acclimation (see section 3), and directly affects the input signal to UVR8 and CRYs as phenolics screen UV-B and UV-A radiation. A time constant of the order of one day or longer can be expected based on the rate of accumulation of flavonoids and phenolic acids (Chappell and Hahlbrock, 1984). The faster feedback affecting UVR8’s state through RUP accumulation (loop labelled 2 in Figure 5) could lead to reversible regulation of responsiveness. This agrees with the observation that upon excitation with broadband UV radiation COP1 bound to UVR8 peaks at 30 min only in non-UV-B-acclimated plants, while HY5 transcript abundance peaks at 90 min and follows very similar time courses in both UV-B-acclimated and non-acclimated plants (Liao et al., 2020, Figures 2, 3). That the time-course of HY5 transcript abundance does not depend on pre-exposure to UV radiation suggests that feedback can buffer downstream signaling from rapid fluctuations in photoreceptor excitation. In addition, the negative feedback on CRYs through BICs (loop labelled 3) affect CRYs signaling in a similar way as RUPs affect UVR8 signaling (Wang et al., 2017). Loops 2 and 3 have HY5 in common and depend each on the action of both UVR8 and CRYs (Tissot and Ulm, 2020). The presence of multiple negative feedback loops and redundant signaling paths is consistent with the observed “fault-tolerance” of the sensory system: lack of either functional UVR8 or functional CRYs is not lethal in full sunlight, while the lack of both is (Rai et al., 2019; Tissot and Ulm, 2020).
Challenges and approaches
To develop applications in plant production and conservation, the main challenge we face is to understand in depth how photomorphogenesis contributes to plant fitness and resilience, and to crop yield and quality. When studying biological systems where complex interactions prevail, one cannot expect reductionist approaches to succeed in providing a satisfactory description of phenomena (Capra and Luisi, 2014), or to significantly contribute to successful applications in agriculture (Sadras et al., 2020). The conditions under which we do experiments put strict boundaries to the range of validity of the results obtained.
As described above, the mechanism of solar UV radiation perception in Arabidopsis depends on complex interactions downstream of photoreceptors and on multiple roles for the individual photoreceptors (Casal, 2013; Morales et al., 2013; Ballaré, 2014; Rai et al., 2019, 2020; Tissot and Ulm, 2020). This suggests that plants, by combining information acquired through different photoreceptors, can differentiate wavelengths and their combinations in much more detail than it has been assumed until now. This implies that in future experiments the design of the environmental conditions used and their detailed characterization and reporting will need to be emphasized much more than in the past.
We need to also pay attention to the fact that plants’ sensory capabilities are subject to natural selection, and consequently that adaptation and acclimation of these capabilities is to be expected as for any other trait contributing to fitness (Gundel et al., 2014; Ballaré and Pierik, 2017). Differences among genotypes and species have been described, but not in the same depth of mechanistic detail as in Arabidopsis (Tossi et al., 2019). Both UVR8 and CRYs are ubiquitous in plants, while the number of copies of UVR8 and CRYs varies among species (Perrotta et al., 2001; Fernández et al., 2016). In the case of UVR8, many species have two copies, but it is not yet known if these copies differ in function (Tossi et al., 2019). Differences in same-generation- and trans-generational responses to UV radiation have been reported for Vicia faba accessions (Yan et al., 2019), but not the mechanisms involved. A possible mechanism could be UVR8-mediated inhibition of DNA methylation (Jiang et al., 2021). Increased emphasis on studying a breadth of species and genotypes would help in understanding the roles of UV perception in plant’s fitness in different habitats.
In experiments, UV treatments can be additive (enhancement) or subtractive (attenuation), that is use of different UV-radiation sources for different treatments versus use of different UV-absorbing filters for the different treatments (Aphalo et al., 2012). At least in principle, both approaches can be used in growth chambers, greenhouses, and outdoors. However, a UV-subtractive approach in a growth chamber is possible only if the chamber is a sun simulator, and in a greenhouse only if its cladding is UV-transparent, requirements that are very seldom fulfilled. Outdoors, UV-enhancement with lamps, results always in higher exposure than the natural one, and the maximum fractional enhancement achievable can be constrained by lamp’s output. In all cases, realistic UV treatments need not only take into account UV irradiance, but the natural balance among wavelengths in the daylight spectrum, as well as timing of UV exposure, both within the day and in relation to plant development. The key point is awareness of what is involved so as to avoid the misinterpretation of results.
The UV radiation sources most commonly used for additive treatments are special fluorescent tubes. Commercial names for these lamps can be easily misleading. Those called broad-band “UV-B lamps” can emit as much UV-A radiation as they emit UV-B radiation (Aphalo et al., 2012) while of those sold as “UV-A lamps” some emit predominantly or only UV-Alw radiation, while others emit predominantly UV-Asw radiation and only some types emit in both the UV-Alw and UV-Asw regions (Supplemental Figure S1). In the case of narrow-band UV-B lamps, the difficulties are fewer. Because of the limited radiation output of the lamps, outdoors it is currently impossible to increase solar UV-A irradiance by more than ca. 5%, while this constraint does not affect UV-B radiation supplementation (Aphalo et al., 2012). This difference stems from the lower UV-B- than UV-A irradiance in sunlight. High-power LEDs for wavelengths equal or longer than 365 nm are readily available at low cost. Those emitting at wavelengths shorter than 365 nm remain inefficient and expensive, restricting their use to the irradiation of a few plants at a time. Xenon-arc lamps although emitting a sun-like spectrum are expensive and fragile, therefore used only in small solar-simulators. Sun-simulation in growth rooms has been based on the simultaneous use of an array of different lamp types plus filters.
Given that most UV-fluorescent tubes emit over a broad range of wavelengths, they need to be used together with UV-absorbing filters to create pairs of treatments and controls differing only in the wavelengths of interest. The enhanced UV-B treatment in outdoor supplementation studies is compared with the misnamed “UV-A control,” which uses the same UV-B lamps but filtered to block UV-B radiation (Middleton and Teramura, 1993; Newsham et al., 1996). The evidence these controls provide for or against UV-A radiation effects is very weak, as these experiments have lacked a control with energized lamps filtered to remove both UV-B and UV-A radiation, which is needed to distinguish the effect of the small UV-A radiation enhancement from other side effects of the lamps. As the daily UV-A enhancement in these controls has been 0.5%–2% of solar UV-A irradiance (Cooley et al., 2000a; Tegelberg et al., 2001), it has seemed unwarranted to assume that this very small enhancement can explain the effect of the filtered UV-B lamps (Newsham et al., 1996). In spite of these limitations, some studies have wrongly interpreted the difference in plant responses between these “UV-A controls” and a control with lamps switched off as demonstrating an effect of UV-A radiation (e.g. Tegelberg et al., 2002; Bernal et al., 2015). On the other hand, the rather consistently observed responses of growth and morphology under UV-B lamps filtered to remove UV-B radiation have remained puzzling since they were discussed in detail by Cooley et al. (2000a). Future UV-A supplementation experiments using lamps and including all the necessary controls could help unravel the drivers behind plant responses observed in “UV-A controls” (Verdaguer et al., 2017), which could be mediated by UV-Asw (Supplemental Figure S1, PET).
Studying responses to UV radiation in field experiments lasting months is challenging due to the variability of weather conditions including cloudiness and solar irradiance that makes replication in time desirable. A middle-ground approach between unrealistic controlled environment experiments and field experiments is the use of sun simulators in which radiation can mimic natural sunlight while controlling other environmental factors such as temperature, humidity, and wind (Rai et al., 2019). An additional approach useful to study transient and short-term responses to solar radiation is to grow plants indoors but to apply treatments outdoors using UV exclusion filters in sunlight (Morales et al., 2013, 2015; Coffey et al., 2017; Rai et al., 2020). It is also important to compare responses to UV exposure in plants grown in the absence of UV radiation with those in plants grown under UV treatments applied continuously, for example since germination, as responses can differ markedly (Rai et al., 2019). Restrictions imposed by regulations on the cultivation of transgenic plants outdoors create difficulties for field experiments resulting in delays and expenses that depend on the country where the research is done (MacKelprang and Lemaux, 2020).
While we need research done under ecologically relevant conditions and aiming at answering ecological questions, such research is greatly facilitated by the knowledge of molecular mechanisms and signaling networks obtained in laboratory experiments. Photobiological research in well-designed artificial contexts is very efficient at identifying molecular players, regulation mechanisms, and points of interaction, while understanding how regulatory interactions and signaling contribute to fitness or crop performance can only be assessed in a realistic environmental context.
Once we take into account that epigenetics, plant hormones, epidermal screening, growth, and morphology are all affected by photoreceptor-mediated responses, the number of possible mechanisms of regulation and paths for interaction and feedback grows dramatically, involving even optics of plant tissues and organs, and light attenuation in canopies. From an ecological perspective, all these interactions can be relevant as they could contribute to plants’ fitness, highlighting the need of multiple experimental approaches, including field experiments and multiple generations of plants, when studying phenotypic plasticity to solar radiation. In practice, only cross-disciplinary collaboration and open-minded scientific dialog will allow us to make good progress.
OUTSTANDING QUESTIONS
Does UVR8 action extend into the UV-A region similarly in all plant species?
What are the direct and indirect roles of cry-dash, PHOTs, and phytochromes inthe perception of solar UV radiation by plants?
How does UV perception through UVR8 and CRYs affect signaling and crosstalk downstream of other plant photoreceptors?
What are the relative contributions of the accumulation of screening pigments and protein-based signaling feedback towards the tuning of perception of different wavelength within the UV waveband?
How does perception of solar UV radiation through photoreceptors, i.e., as an information-carrying cue, contribute to plant fitness? Do these contributions to fitness go any farther than tolerance of exposure to UV radiation itself?
Concluding remarks
Photoreceptor-driven plant responses have been extensively studied and reported for UV-B, blue, and red/far-red spectral regions. A gap in knowledge had remained due to the lack of an equivalent research effort in the UV-A region of the solar spectrum. Recent studies showing that perception of solar UV-Asw by plants is mediated by the “UV-B” photoreceptor UVR8 and the complexity of signaling interactions make it necessary to revise accepted views on the perception of solar UV radiation by plants and the role it may play in plant fitness (see Outstanding Questions). Further research is required to assess the direct and indirect roles of different photoreceptors in UV-induced changes in gene expression, morphology, growth, photosynthetic performance, and metabolite profiles. The recent studies showing that plants differentiate between UV-Asw and UV-Alw and differently respond to these bands serves as a base for future studies, in which it will be required to separately measure and/or manipulate UV-Asw and UV-Alw, as we now know that in sunlight these bands are predominantly perceived through different photoreceptors.
Supplemental data
Supplemental Figure S1. Commonly used UV light sources.
Supplementary Material
Acknowledgments
The authors thank Anders V. Lindfors for making available the data set of simulated solar spectra for Helsinki used in Figure 1. They also thank Mikael Brosché, Tarja Lehto, and T. Matthew Robson for providing feedback on the manuscript. They acknowledge fruitful discussions with Lars Olof Björn, Jorge J. Casal, Ariel Novoplansky, Victor O. Sadras, Nicolas Tissot, and Roman Ulm that helped develop the ideas discussed in the manuscript.
Funding
This work was supported by a PhD Fellowship from Doctoral Programme in Plant Sciences (University of Helsinki), EMBO Short-Term Fellowship (ASTF 570-2016), Finnish Cultural Foundation, and Alfred Kordelin Foundation to N.R.
Conflict of interest statement. No conflicts of interest.
N.R. conceptualized the layout of the manuscript. N.R. and P.J.A. wrote the manuscript with contributions from L.O.M. All three authors revised and commented on the whole manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Neha Rai (neha.rai@unige.ch).
References
- Aphalo PJ, Albert A, McLeod AR, Heikkilä A, Gómez I, López Figueroa F, Robson TM, Strid Å (2012) Manipulating UV radiation. InAphalo PJ, Albert A, Björn LO, McLeod AR, Robson TM, Rosenqvist E, eds, Beyond the Visible: A Handbook of Best Practice in Plant UV Photobiology , Ed 1,University of Helsinki, Department of Biosciences, Division of Plant Biology, pp 35–70 [Google Scholar]
- Aphalo PJ (2015) The r4photobiology suite: spectral irradiance. UV4Plants Bull 1:21–29 [Google Scholar]
- Ballaré CL (2014) Light regulation of plant defense. Annu Rev Plant Biol 65:335–363 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Pierik R (2017) The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ 40:2530–2543 [DOI] [PubMed] [Google Scholar]
- Banerjee R, Schleicher E, Meier S, Viana RM, Pokorny R, Ahmad M, Bittl R, Batschauer A (2007) The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J Biol Chem 282:14916–14922 [DOI] [PubMed] [Google Scholar]
- Barnes PW, Flint SD, Tobler MA, Ryel RJ (2016a) Diurnal adjustment in ultraviolet sunscreen protection is widespread among higher plants. Oecologia 181:55–63 [DOI] [PubMed] [Google Scholar]
- Barnes PW, Tobler MA, Keefover-Ring K, Flint SD, Barkley AE, Ryel RJ, Lindroth RL (2016b) Rapid modulation of ultraviolet shielding in plants is influenced by solar ultraviolet radiation and linked to alterations in flavonoids. Plant Cell Environ 39:222–230 [DOI] [PubMed] [Google Scholar]
- Barnes PW, Ryel RJ, Flint SD (2017) UV screening in native and non-native plant species in the tropical alpine: Implications for climate change-driven migration of species to higher elevations. Front Plant Sci 8:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal M, Verdaguer D, Badosa J, Abadía A, Llusià J, Peñuelas J, Núñez-Olivera E, Llorens L (2015) Effects of enhanced UV radiation and water availability on performance, biomass production and photoprotective mechanisms of Laurus nobilis seedlings. Environ Exp Bot 109:264–275 [Google Scholar]
- Bernula P, Crocco CD, Arongaus AB, Ulm R, Nagy F, Viczián A (2017) Expression of the UVR8 photoreceptor in different tissues reveals tissue-autonomous features of UV-B signalling. Plant Cell Environ 40:1104–1114 [DOI] [PubMed] [Google Scholar]
- Bidel LPR, Chomicki G, Bonini F, Mondolot L, Soulé J, Coumans M, La Fisca P, Baissac Y, Petit V, Loiseau A, et al. (2015) Dynamics of flavonol accumulation in leaf tissues under different UV-B regimes in Centella asiatica (Apiaceae). Planta 242:545–559 [DOI] [PubMed] [Google Scholar]
- Binkert M, Ulm R (2017) UV-B signal transduction from photoperception to response. InJordan BR, ed., UV-B Radiation and Plant Life: Molecular Biology to Ecology, CABI, pp 130–143 [Google Scholar]
- Björn LO (2015) History ultraviolet-A, B, and C. UV4Plants Bull 1:17–18 [Google Scholar]
- Briggs WR, Huala E (1999) Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol 15:33–62 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7:204–210 [DOI] [PubMed] [Google Scholar]
- Brodersen CR, Vogelmann TC (2010) Do changes in light direction affect absorption profiles in leaves? Funct Plant Biol 37:403–412 [Google Scholar]
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA 102:18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, Headland LR, Jenkins GI (2009) UV-B action spectrum for UVR8-mediated HY5 transcript accumulation in Arabidopsis. Photochem Photobiol 85:1147–1155 [DOI] [PubMed] [Google Scholar]
- Capra F, Luisi PL (2014) The systems view of life: A unifying vision. Cambridge: Cambridge University Press (doi: 10.1017/CBO9780511895555) [Google Scholar]
- Casal JJ (2013) Photoreceptor signaling networks in plant responses to shade. Ann Rev Plant Biol 64:403–427 [DOI] [PubMed] [Google Scholar]
- Chappell J, Hahlbrock K (1984) Transcription of plant defence genes in response to UV light or fungal elicitor. Nature 311:76–78 [Google Scholar]
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen L-O, van der Horst GTJ, Batschauer A, Ahmad M (2011) The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol 62:335–364 [DOI] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38:87–117 [DOI] [PubMed] [Google Scholar]
- Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, et al. , (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335:1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Blackwood L, Petersen J, Sullivan S (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol 56:401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey A, Prinsen E, Jansen MAK, Conway J (2017) The UVB photoreceptor UVR8 mediates accumulation of UV-absorbing pigments, but not changes in plant morphology, under outdoor conditions. Plant Cell Environ 40:2250–2260 [DOI] [PubMed] [Google Scholar]
- Cooley NM, Holmes MG, Attridge TH (2000a) Growth and stomatal responses of temperate meadow species to enhanced levels of UV-A and UV-B+A radiation in the natural environment. J Photochem Photobiol B Biol 57:179–185 [DOI] [PubMed] [Google Scholar]
- Cooley NM, Truscott HMF, Holmes MG, Attridge TH (2000b) Outdoor ultraviolet polychromatic action spectra for growth responses of Bellis perennis and Cynosurus cristatus. J Photochem Photobiol B Biol 59:64–71 [DOI] [PubMed] [Google Scholar]
- Day TA, Martin G, Vogelmann TC (1993) Penetration of UV-B radiation in foliage: evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environ 16:735–741 [Google Scholar]
- Demkura PV, Ballaré CL (2012) UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant 5:642–652 [DOI] [PubMed] [Google Scholar]
- Díaz-Ramos LA, O’Hara A, Kanagarajan S, Farkas D, Strid Å, Jenkins GI (2018) Difference in the action spectra for UVR8 monomerisation and HY5 transcript accumulation in Arabidopsis. Photochem Photobiol Sci 17:1108–1117 [DOI] [PubMed] [Google Scholar]
- Dotto M, Casati P (2017) Developmental reprogramming by UV-B radiation in plants. Plant Sci 264:96–101 [DOI] [PubMed] [Google Scholar]
- Duell-Pfaff N, Wellmann E (1982) Involvement of phytochrome and a blue light photoreceptor in UV-B induced flavonoid synthesis in parsley (Petroselinum hortense Hoffm.) cell suspension cultures. Planta 156:213–217 [DOI] [PubMed] [Google Scholar]
- Emde C, Buras‐Schnell R, Kylling A, Mayer B, Gasteiger J, Hamann U, Kylling J, Richter B, Pause C, Dowling T, et al. (2016) The libRadtran software package for radiative transfer calculations (version 2.0.1). Geosci Model Dev 9:1647–1672 [Google Scholar]
- Favory J-JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández MB, Tossi V, Lamattina L, Cassia R (2016) A comprehensive phylogeny reveals functional conservation of the UV-B photoreceptor UVR8 from green algae to higher plants. Front Plant Sci 7: 1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina J, Casadevall R, Abdelgawad H, Prinsen E, Markakis MN, Beemster GTS, Casati P (2017) UV-B inhibits leaf growth through changes in growth regulating factors and Gibberellin levels. Plant Physiol 174:1110–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay KMW, Jenkins GI (2016) Regulation of UVR8 photoreceptor dimer/monomer photo-equilibrium in Arabidopsis plants grown under photoperiodic conditions. Plant Cell Environ 39:1706–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26:471–478 [DOI] [PubMed] [Google Scholar]
- Fuglevand G, Jackson JA, Jenkins GI (1996) UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8:2347–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9:1353–1365 [DOI] [PubMed] [Google Scholar]
- Gorton HL (2010) Biological action spectra, Photobiol Sci Online (KC Smith, ed.) Am Soc Photobiol http://photobiology.info/Gorton.html
- Gruber H, Heijde M, Heller W, Albert A, Seidlitz HK, Ulm R (2010) Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc Natl Acad Sci USA 107:20132–20137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel PE, Pierik R, Mommer L, Ballaré CL (2014) Competing neighbors: Light perception and root function. Oecologia 176:1–10 [DOI] [PubMed] [Google Scholar]
- Guo H, Duong H, Ma N, Lin C (1999) The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light-dependent post-transcriptional mechanism. Plant J 19:279–287 [DOI] [PubMed] [Google Scholar]
- Hayes S, Velanis CN, Jenkins GI, Franklin KA (2014) UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc Natl Acad Sci USA 111:11894–11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijde M, Ulm R (2013) Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc Natl Acad Sci USA 110:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanowicz P, Banaś AK, Sztatelman O, Gabryś H, Łabuz J (2019) UV-B induces chloroplast movements in a phototropin-dependent manner. Front Plant Sci 10:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg É, Jansen MAK, Strid Å (. 2013) UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci 18:107.– [DOI] [PubMed] [Google Scholar]
- Hideg É, Strid A (2017) The effects of UV-B on the biochemistry and metabolism of plants. InJordan BR, ed., UV-B Radiation and Plant Life: Molecular Biology to Ecology, CABI, pp 90–110. [Google Scholar]
- Hoecker U (2017) The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr Opin Plant Biol 37:63–69 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Keiller DR (2002) Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species. Plant Cell Environ 25:85–93 [Google Scholar]
- Holopainen JK, Kivimäenpää M, Julkunen-Tiitto R (2018) New light for phytochemicals. Trends Biotechnol 36:7–10 [DOI] [PubMed] [Google Scholar]
- Holtkotte X, Ponnu J, Ahmad M, Hoecker U (2017) The blue light-induced interaction of cryptochrome 1 with COP1 requires SPA proteins during Arabidopsis light signaling. PLOS Genet 13:e1007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, Deng XW (2013) Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci USA 110:16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner JC, Olteanu VA, Hetherington AJ, Coupel-Ledru A, Sun P, Pridgeon AJ, Jones GS, Oates M, Williams TA, Maathuis FJM, et al. (2019) Short- and long-term effects of UVA on Arabidopsis are mediated by a novel cGMP phosphodiesterase. Curr Biol 29:2580–2585.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MA, Urban O (2019) Plant Responses to UV-B Radiation. eLS. John Wiley & Sons, Ltd, Chichester, UK, pp 1–11 [Google Scholar]
- Jenkins GI (2014) Structure and function of the UV-B photoreceptor UVR8. Curr Opin Struct Biol 29:52–57 [DOI] [PubMed] [Google Scholar]
- Jenkins GI (2017) Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ 40:2544–2557 [DOI] [PubMed] [Google Scholar]
- Jiang J, Liu J, Sanders D, Qian S, Ren W, Song J, Liu F, Zhong X (2021) UVR8 interacts with de novo DNA methyltransferase and suppresses DNA methylation in Arabidopsis. Nat Plants 7: 184–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserli E, Jenkins GI (2007) UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19:2662–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand Å. (2007). Genome‐wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 144:1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem K, Holub P, Štroch M, Nezval J, Špunda V, Tříska J, Jansen MAK, Robson TM, Urban O (2015) Ultraviolet and photosynthetically active radiation can both induce photoprotective capacity allowing barley to overcome high radiation stress. Plant Physiol Biochem 93:74–83 [DOI] [PubMed] [Google Scholar]
- Kolb CA, Schreiber U, Gademann R, Pfundel EE (2005) UV-A screening in plants determined using a new portable fluorimeter. Photosynthetica 43:371–377 [Google Scholar]
- Kolb CA, Pfündel EE (2005) Origins of non-linear and dissimilar relationships between epidermal UV absorbance and UV absorbance of extracted phenolics in leaves of grapevine and barley. Plant Cell Environ 28:580–590 [Google Scholar]
- Krauss P, Markstädter C, Riederer M (1997) Attenuation of UV radiation by plant cuticles from woody species. Plant Cell Environ 20:1079–1085 [Google Scholar]
- Lau K, Podolec R, Chappuis R, Ulm R, Hothorn M (2019) Plant photoreceptors and their signaling components compete for COP 1 binding via VP peptide motifs. EMBO J 38:e102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ren H, Kundu M, Liu Z, Zhong FW, Wang L, Gao J, Zhong D (2020) A leap in quantum efficiency through light harvesting in photoreceptor UVR8. Nat Commun 11:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Mei S, Shi C, Yang Y, Peng Y, Ma L, Wang F, Li X, Huang X, Yin Y, et al. (2018) UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis. Dev Cell 44:512–523 [DOI] [PubMed] [Google Scholar]
- Liao X, Liu W, Yang H-Q, Jenkins GI (2020) A dynamic model of UVR8 photoreceptor signalling in UV-B-acclimated Arabidopsis. New Phytologist 227:857–866. [DOI] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR (1995) Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc Natl Acad Sci USA 92:8423–8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Cashmore AR (1996) Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J 10:893–902 [DOI] [PubMed] [Google Scholar]
- Lindfors A, Heikkilä A, Kaurola J, Koskela T, Lakkala K (2009) Reconstruction of solar spectral surface UV irradiances using radiative transfer simulations. Photochem Photobiol 85:1233–1239 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7:473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hodgson DW, Campbell TJ (2003) Blue light signaling through the cryptochromes and phototropins. So that’s what the blues is all about. Plant Physiol 133:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Nittler P, Koskie K (2020) The continuing arc toward phototropic enlightenment. J Exp Bot 71:1652–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKelprang R, Lemaux PG (2020) Genetic engineering and editing of plants: An analysis of new and persisting questions. Annu Rev Plant Biol 71:659–687 [DOI] [PubMed] [Google Scholar]
- Mazza CA, Ballaré CL (2015) Photoreceptors UVR8 and phytochrome B cooperate to optimize plant growth and defense in patchy canopies. New Phytol 207:4–9 [DOI] [PubMed] [Google Scholar]
- McCree KJ (1972) Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric Meteorol 10:443–453 [Google Scholar]
- Middleton EM, Teramura AH (1993) The role of flavonol glycosides and carotenoids in protecting soybean from Ultraviolet-B damage. Plant Physiol 103:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LO, Tegelberg R, Brosché M, Keinänen M, Lindfors A, Aphalo PJ (2010) Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol 30:923–934 [DOI] [PubMed] [Google Scholar]
- Morales LO, Tegelberg R, Brosché M, Lindfors A, Siipola S, Aphalo PJ (2011) Temporal variation in epidermal flavonoids due to altered solar UV radiation is moderated by the leaf position in Betula pendula. Physiol Plant 143:261–270 [DOI] [PubMed] [Google Scholar]
- Morales LO, Brosché M, Vainonen J, Jenkins GI, Wargent JJ, Sipari N, Strid Å, Lindfors AV, Tegelberg R, Aphalo PJ (2013) Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation. Plant Physiol 161:744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LO, Brosché M, Vainonen JP, Sipari N, Lindfors AV, Strid Å, Aphalo PJ (2015) Are solar UV-B- and UV-A-dependent gene expression and metabolite accumulation in Arabidopsis mediated by the stress response regulator RADICAL-INDUCED CELL DEATH1? Plant Cell Environ 38:878–891 [DOI] [PubMed] [Google Scholar]
- Moriconi V, Binkert M, Costigliolo C, Sellaro R, Ulm R, Casal JJ (2018) Perception of sunflecks by the UV-B photoreceptor UV RESISTANCE LOCUS8. Plant Physiol 177:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham KK, McLeod AR, Greenslade PD, Emmett BA (1996) Appropriate controls in outdoor UV-B supplementation experiments. Glob Chang Biol 2:319–324 [Google Scholar]
- Pedmale UV, Huang SC, Zander M, Cole BJ, Hetzel J, Ljung K, Reis PAB, Sridevi P, Nito K, Nery JR, et al. (2016) Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164:233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta G, Yahoubyan G, Nebuloso E, Renzi L, Giuliano G (2001) Tomato and barley contain duplicated copies of cryptochrome 1. Plant Cell Environ 24:991–998 [Google Scholar]
- Pescheck F, Bilger W (2019) High impact of seasonal temperature changes on acclimation of photoprotection and radiation-induced damage in field grown Arabidopsis thaliana. Plant Physiol Biochem 134:129–136 [DOI] [PubMed] [Google Scholar]
- Pieristè M, Neimane S, Solanki T, Nybakken L, Jones AG, Forey E, Chauvat M, Ņečajeva J, Robson TM (2020) Ultraviolet radiation accelerates photodegradation under controlled conditions but slows the decomposition of senescent leaves from forest stands in southern Finland. Plant Physiol Biochem 146:42–54 [DOI] [PubMed] [Google Scholar]
- Podolec R, Ulm R (2018) Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol 45:18–25 [DOI] [PubMed] [Google Scholar]
- Ponnu J, Riedel T, Penner E, Schrader A, Hoecker U (2019) Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc Natl Acad Sci USA 116:27133–27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. (2020) A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (https://www.r-project.org/)
- Rai N, Neugart S, Yan Y, Wang F, Siipola SM, Lindfors A V, Winkler JB, Albert A, Brosché M, Lehto T, et al. (2019) How do cryptochromes and UVR8 interact in natural and simulated sunlight? J Exp Bot 70:4975–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N, O’Hara A, Farkas D, Safronov O, Ratanasopa K, Wang F, Lindfors AV, Jenkins GI, Lehto T, Salojärvi J, et al. (2020) The photoreceptor UVR8 mediates the perception of both UV‐B and UV‐A wavelengths up to 350 nm of sunlight with responsivity moderated by cryptochromes. Plant Cell Environ 43:1513–1527 [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory J-J, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106 [DOI] [PubMed] [Google Scholar]
- Robson TM, Aphalo PJ, Banaś AK, Barnes PW, Brelsford CC, Jenkins GI, Kotilainen TK, Łabuz J, Martínez-Abaigar J, Morales LO, et al. (2019) A perspective on ecologically relevant plant-UV research and its practical application. Photochem Photobiol Sci 18:970–988 [DOI] [PubMed] [Google Scholar]
- Sadras V, Alston J, Aphalo P, Connor D, Denison RF, Fischer T, Gray R, Hayman P, Kirkegaard J, Kirchmann H, et al. (2020) Making science more effective for agriculture. Adv Agron 163:153–177 [Google Scholar]
- Siipola SM, Kotilainen T, Sipari N, Morales LO, Lindfors AV, Robson TM, Aphalo PJ (2015) Epidermal UV-A absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ 38:941–952 [DOI] [PubMed] [Google Scholar]
- Solanki T, Aphalo PJ, Neimane S, Hartikainen SM, Pieristè M, Shapiguzov A, Porcar-Castell A, Atherton J, Heikkilä A, Robson TM (2019) UV-screening and springtime recovery of photosynthetic capacity in leaves of Vaccinium vitis-idaea above and below the snow pack. Plant Physiol Biochem 134:40–52 [DOI] [PubMed] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ (2001) The effects of long-term elevated UV-B on the growth and phenolics of field-grown silver birch (Betula pendula). Glob Chang Biol 7:839–848 [Google Scholar]
- Tegelberg R, Aphalo PJ, Julkunen-Tiitto R (2002) Effects of long-term, elevated ultraviolet-B radiation on phytochemicals in the bark of silver birch (Betula pendula). Tree Physiol 22:1257–1263 [DOI] [PubMed] [Google Scholar]
- Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R (2013) The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11:e0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot N, Ulm R (2020) Cryptochrome-mediated blue-light signalling modulates UVR8 photoreceptor activity and contributes to UV-B tolerance in Arabidopsis. Nat Commun 11:1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi V, Lamattina L, Jenkins GI, Cassia RO (2014) Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol 164:2220–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi VE, Regalado JJ, Iannicelli J, Laino LE, Burrieza HP, Escandón AS, Pitta-Álvarez SI (2019) Beyond Arabidopsis: Differential UV-B response mediated by UVR8 in diverse species. Front Plant Sci 10:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Tilbrook K, Fierro AC, Marchal K, Poelman D, Van Der Straeten D, Ulm R (2014) Photoreceptor-mediated bending towards UV-B in Arabidopsis. Mol Plant 7:1041–1052 [DOI] [PubMed] [Google Scholar]
- Vanhaelewyn L, Prinsen E, Van Der Straeten D, Vandenbussche F (2016) Hormone-controlled UV-B responses in plants. J Exp Bot 67:4469–4482 [DOI] [PubMed] [Google Scholar]
- Vanhaelewyn L, Viczián A, Prinsen E, Bernula P, Serrano AM, Arana MV, Ballaré CL, Nagy F, van der Straeten D, Vandenbussche F (2019) Differential UVR8 signal across the stem controls UV-B-induced inflorescence phototropism. Plant Cell 31:2070–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Bilger W, Mühlbauer T, Brummet W, Winter K (1996) Diurnal changes in flavonoids. Plant Physiol 148:478–482 [Google Scholar]
- Verdaguer D, Jansen MAKK, Llorens L, Morales LO, Neugart S (2017) UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci 255:72–81 [DOI] [PubMed] [Google Scholar]
- Wang F, Robson TM, Casal JJ, Shapiguzov A, Aphalo PJ (2020) Contributions of cryptochromes and phototropins to stomatal opening through the day. Funct Plant Biol 47:226–238 [DOI] [PubMed] [Google Scholar]
- Wang Q, Lin C (2020) Mechanisms of cryptochrome-mediated photoresponses in plants. Annu Rev Plant Biol 71:103–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zuo Z, Wang X, Gu L, Yoshizumi T, Yang Z, Yang L, Liu Q, Liu W, Han Y-J, et al. (2016) Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 354:343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lu X, Li L, Lian H, Mao Z, Xu P, Guo T, Xu F, Du S, Cao X, et al. (2018) Photoexcited CRYPTOCHROME1 interacts with dephosphorylated BES1 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis. Plant Cell 30:1989–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Q, Han Y-J, Liu Q, Gu L, Yang Z, Su J, Liu B, Zuo Z, He W, et al. (2017) A CRY-BIC negative-feedback circuitry regulating blue light sensitivity of Arabidopsis. Plant J 92:426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, et al. (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686 [Google Scholar]
- Wu G, Spalding EP (2007) Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc Natl Acad Sci USA 104:18813–18818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Stoddard FL, Neugart S, Sadras VO, Lindfors A, Morales LO, Aphalo PJ (2019) Responses of flavonoid profile and associated gene expression to solar blue and UV radiation in two accessions of Vicia faba L. from contrasting UV environments. Photochem Photobiol Sci 18:434–447 [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu B, Su J, Liao J, Lin C, Oka Y (2017) Cryptochromes orchestrate transcription regulation of diverse blue light responses in plants. Photochem Photobiol 93:112–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, Ulm R (2017) How plants cope with UV-B: from perception to response. Curr Opin Plant Biol 37:42–48 [DOI] [PubMed] [Google Scholar]
- Yu X, Klejnot J, Zhao X, Shalitin D, Maymon M, Yang H, Lee J, Liu X, Lopez J, Lin C (2007) Arabidopsis cryptochrome 2 completes its posttranslational life cycle in the nucleus. Plant Cell 19:3146–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Liu H, Klejnot J, Lin C (2010) The cryptochrome blue light receptors. Arab B 8:e0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.