Figure 1.
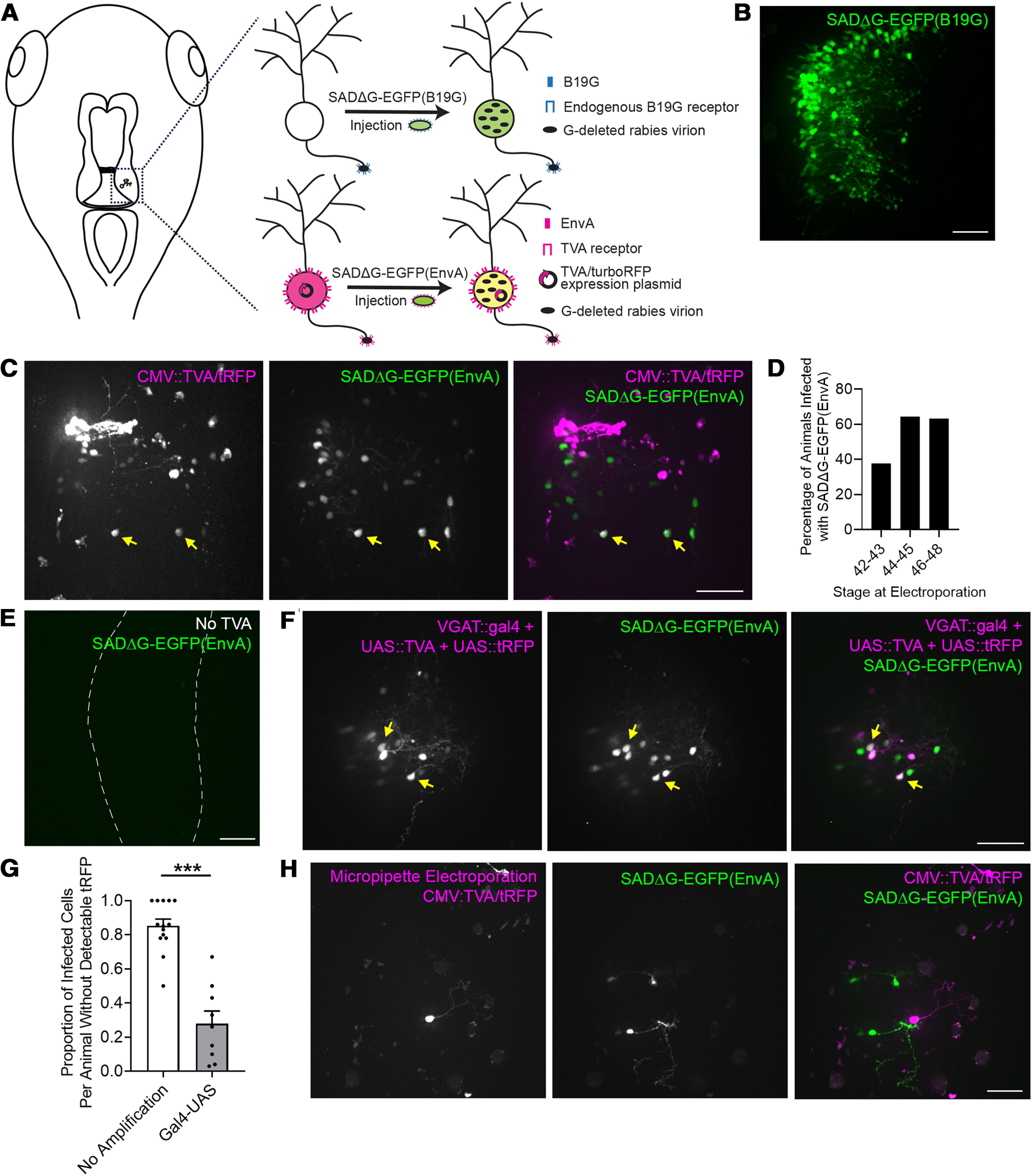
Pseudotyped recombinant rabies virus infects tectal neurons in the Xenopus tadpole. A, Schematic of the labeling strategies using recombinant SAD B19 rabies virus which has the glycoprotein deleted and replaced by EGFP (SADΔG-EGFP), rendering it incapable of transneuronal spread. Infection with B19G phenotypically complemented virus (top) relies on endogenous expression of the B19G receptor. Infection with EnvA pseudotyped virus (bottom) requires exogenous expression of its receptor, TVA, before viral injection. Co-labeling TVA-transfected neurons with tRFP allows them to be identified. Viral injections were made in the optic tectum, which is marked by a dashed box in the drawing to the left. B, SADΔG-EGFP(B19G) virus infects tectal neurons. Confocal Z-projection collected in vivo through the injected optic tectal lobe shows widespread virally-mediated expression of EGFP in infected neurons. C, SADΔG-EGFP(EnvA) virus infects tectal neurons transfected with TVA. The right optic tectal lobe was transfected with CMV::TVA/tRFP by whole-brain electroporation and injected with SADΔG-EGFP(EnvA) virus 4 d later. Confocal Z-projections collected in vivo through the optic tectal lobe electroporated with CMV::TVA/tRFP (magenta) and injected with SADΔG-EGFP(EnvA) virus (green). Neurons which co-express TVA/tRFP and viral EGFP are marked by yellow arrows. The remaining EGFP-expressing neurons lack detectable tRFP expression and are presumably invisible TVA-expressing neurons. D, Viral infection efficiency varies with developmental stage. Tadpoles at stages 42–43 (n = 16 tadpoles), 44–45 (n = 28 tadpoles), or 46–48 (n = 65 tadpoles) were electroporated with CMV::TVA/tRFP using whole-brain electroporation. Four days later, the transfected tectal lobe was injected with SADΔG-EGFP(EnvA) virus. The percentage of animals with EGFP-expressing neurons was highest between stages 44 and 48. E, Infection with SADΔG-EGFP(EnvA) virus requires TVA. Confocal Z-projection collected in vivo through an optic tectal lobe injected with SADΔG-EGFP(EnvA) shows no infected neurons in the absence of TVA electroporation. F, SADΔG-EGFP(EnvA) infects tectal neurons transfected with TVA driven by the VGAT promoter. The right optic tectal lobe was transfected with VGAT::gal4, UAS::TVA, and UAS::tRFP by whole-brain electroporation and injected with SADΔG-EGFP(EnvA) virus 4 d later. Confocal Z-projections collected in vivo through the optic tectal lobe showing electroporated (magenta) and infected (green) tectal neurons. Neurons which co-express TVA/tRFP and viral EGFP are marked by yellow arrows. The remaining EGFP-expressing neurons lack detectable tRFP expression and are invisible TVA-expressing neurons. G, Quantification of the proportion of invisible TVA cells per animal with and without amplification. Amplifying tRFP expression using the gal4-UAS system decreases the proportion of infected, EGFP+ cells that lack detectable tRFP compared with tRFP driven by the CMV promoter without amplification. Data are presented as mean ± SEM overlaid with individual data points (***p < 0.0001, Mann–Whitney test). H, Targeted electroporation of TVA/tRFP does not eliminate invisible TVA-expressing neurons. Micropipette-mediated electroporation was used to limit transfection with TVA/tRFP to one or few neurons in the right optic tectal lobe. Four days later, the electroporated tectal lobe was injected with SADΔG-EGFP(EnvA) virus. Confocal Z-projection collected in vivo through the optic tectal lobe shows that EGFP-expressing infected neurons which lack detectable tRFP are still present. Scale bars: 50 μm.
