Figure 3.
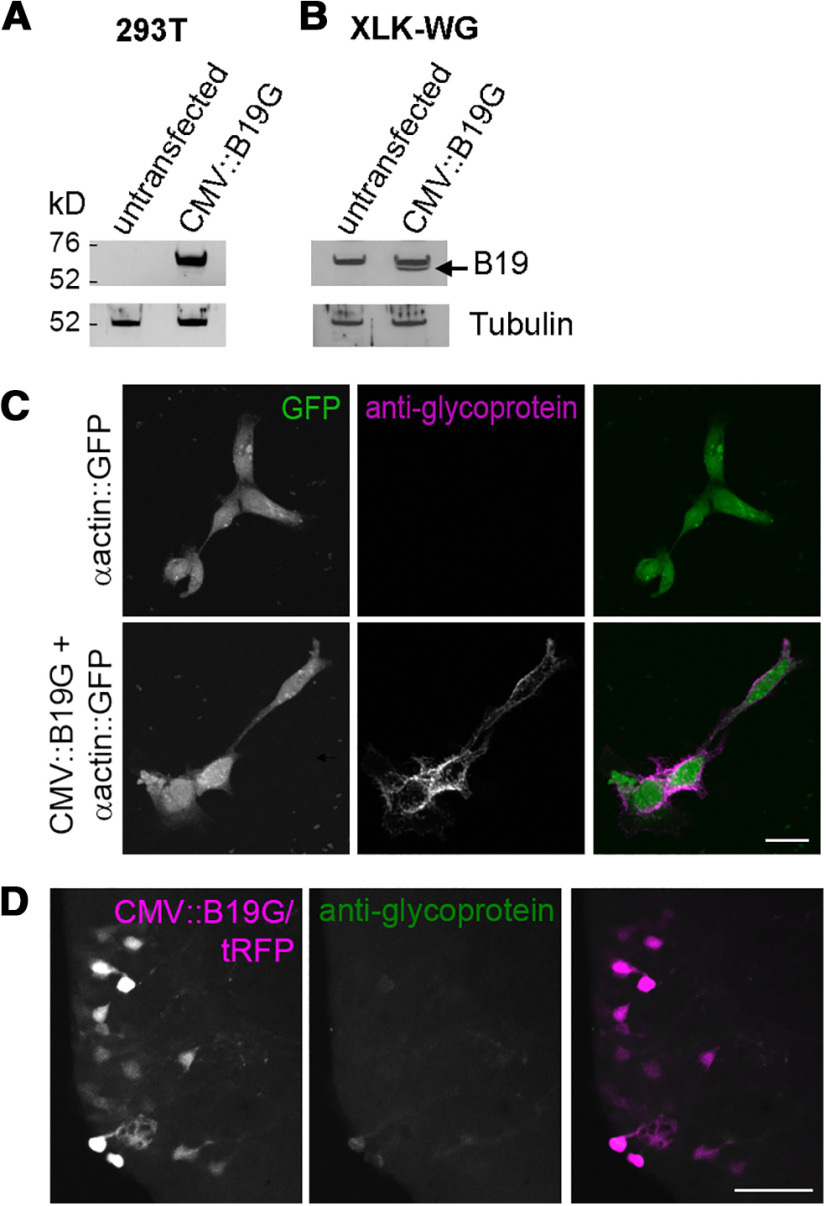
Weak expression of B19G in vivo may explain the lack of transneuronal spread of rabies virus. A, B, Rabies glycoprotein is detected in the membrane fraction of transfected mammalian and Xenopus cell cultures by Western blotting. 293T (A) and XLK-WG Xenopus kidney cells (B) were transfected with B19G and proteins were extracted 24 h later. Membrane fractions were probed for B19G expression with anti-rabies glycoprotein antibody and β-tubulin acted as a loading control. Compared with untransfected cells, specific bands of ∼70 kDa were visible in transfected cells. Specific band in transfected XLK-WG cells is denoted by an arrow (B). C, Rabies glycoprotein is detected on the surface of Xenopus cells in vitro by immunocytochemistry. XLK-WG cells were transfected with GFP alone (top) or B19G and GFP (bottom). Confocal Z-projections of cells transfected with both B19G and GFP show surface expression of B19G by anti-rabies glycoprotein immunocytochemistry (magenta) without permeabilization. In contrast, no anti-rabies glycoprotein immunoreactivity is observed in cells transfected with GFP alone. Scale bar: 20 μm. D, Expression of B19G is very weak in vivo in tectal neurons. Tectal neurons were electroporated with CMV::B19G/tRFP, fixed 3–4 d later, and then immunohistochemistry with anti-rabies glycoprotein was performed. Confocal Z-projection of a 40-μm tissue slice shows very weak immunoreactivity for rabies glycoprotein (green) in B19G/tRFP expressing neurons (magenta). Scale bar: 50 μm.