Figure 2.
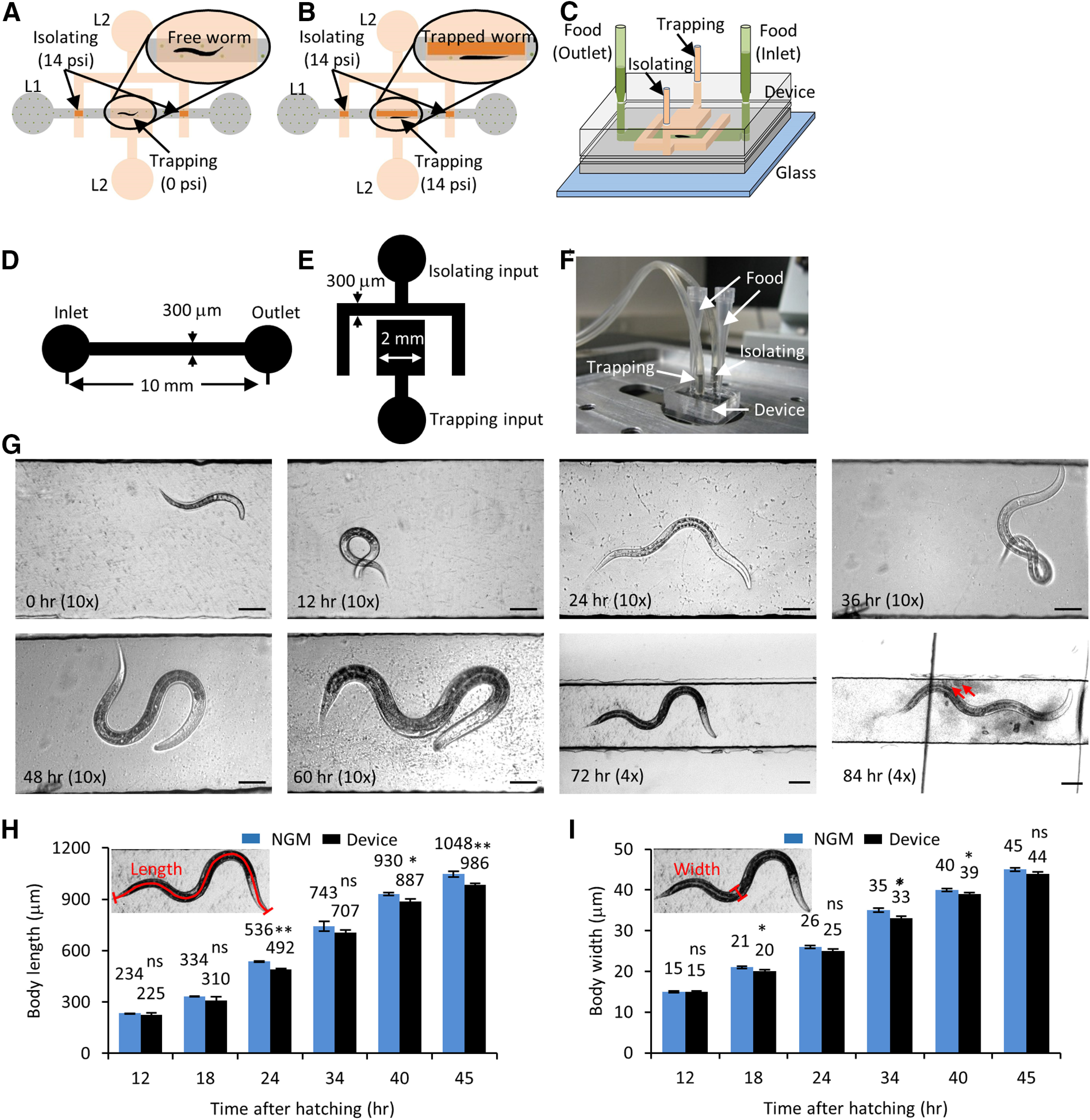
Growth and development of C. elegans are unaffected in the long-term growth and imaging microfluidic device. A, Schematic of the microfluidic device with a flow channel in the bottom layer (L1, gray) and isolating and trapping channels in the control layer on top (L2, orange). The isolating membrane is always kept on, with 14 psi pressure (deflected membrane denoted by dark orange) to restrict the free animal shown in the inset. B, Trap pressure is turned on (14 psi, deflected membrane denoted by dark orange) to immobilize the animal inside the flow channel (inset) during imaging. C, 3D view of the device layout with two pipettes tips (light green) as food inlet and outlet connecting the flow channel (dark green). D, Schematic for the flow layer with channel dimensions. E, Schematic of the control layer with channel dimensions. F, Image of a growth and imaging device connected to compressed nitrogen gas and food supply. G, Images of a C. elegans hermaphrodite growing inside the microfluidic device at 0, 12, 24, 36, 48, 60, 72, and 84 h posthatching. The animal is fed OP50 bacteria and isolated inside the flow channel and imaged with a 4× or 10× objective. The red arrows indicate freshly laid eggs at 84 h. Scale bars: 50 or 100 μm at the 72- and 84-h time points. H, I, jsIs609 animals grown in the device (black) and on regular NGM plates (blue) were used for calculating body length (H) and body diameter (I). The small inset shows the schematic for body length and body diameter from worm images. The average values are mentioned on top of each bar. Data represented as mean ± SEM, n = 12 animals. Statistical significance was evaluated by paired sample t test; *p < 0.05, **p < 0.005, and ns, p > 0.05 when compared between animals grown on NGM and in liquid culture.
