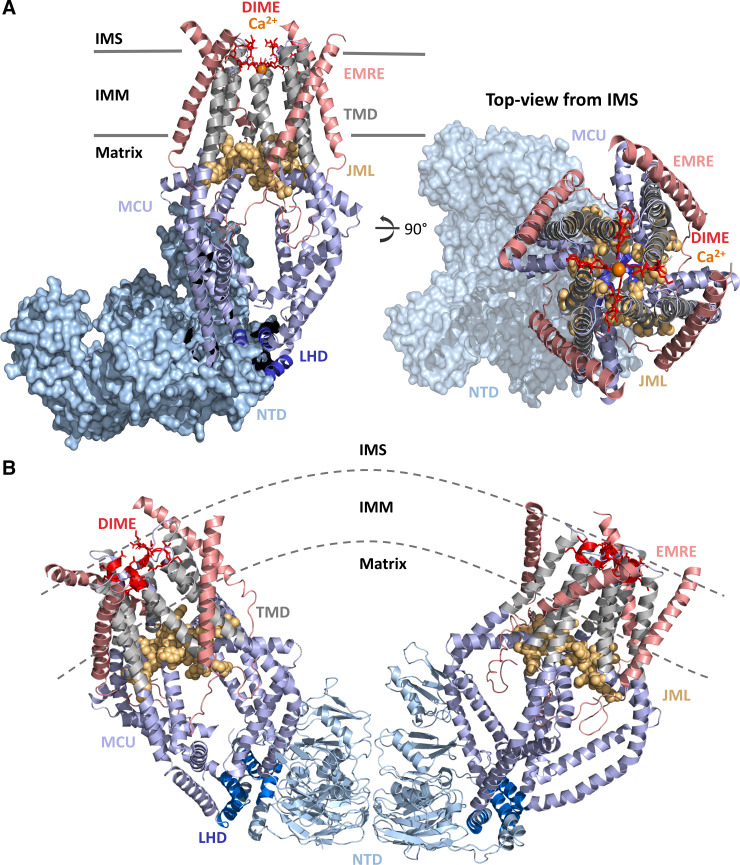
Figure 2.
Structure of the human MCU-essential MCU regulator (EMRE) complex. A: structure of the MCU-EMRE uniplex channel in the presence of Ca2+. Four human MCU subunits (all blue and gray) are in complex with 4 EMRE peptides (salmon). The MCU NH2 and COOH termini are oriented in the matrix. The MCU NTD (light blue surface) adopts a crescent tetramer assembly. The DIME motif (residues 261–264; red sticks) forms the selectivity filter for Ca2+ (orange spheres) near the IMS entrance to the channel. The MCU TMDs (gray cartoons) symmetrically surround the pore. The JML (residues 285–290; light orange space fill) encircles the pore exit and may function as a luminal gate. The LHD (residues 178–188; dark blue cartoon) segregates the MCU NTD from the pore. The four EMRE peptides are oriented with the NH2 termini in the matrix and the COOH termini in the IMS. B: V-shaped dimer of two human MCU-EMRE uniplex channels. The MCU NTDs (light blue cartoon) mediate dimerization of two complexes. Coloring of MCU and EMRE is consistent with A. The structure figures were rendered in PyMOL (Schrodinger LLC) using the 6O5B and 6O58 pdb coordinate files (42). IMM, inner mitochondrial membrane; IMS, intermembrane space; JML, juxtamembrane loop; LHD, linker helix domain; MCU, mitochondrial calcium uniporter; NTD, NH2-terminal domain; TMD, transmembrane domain.