Keywords: fasting, insulin action, insulin secretion, insulin secretion disposition index, small for gestational age
Abstract
The extent to which reduced insulin secretion during prolonged fasting reflects failure to compensate for whole body insulin resistance or a normal adjustment to potentially increased hepatic insulin action is unknown. We examined the effects of 36- versus 12-h fasting on insulin secretion and whole body versus hepatic insulin action in 13 healthy young males. Hepatic glucose production and insulin action were studied using stable isotopes, whereas whole body insulin action and insulin secretion were studied using an intravenous glucose tolerance test (IVGTT) and minimal modeling. Insulin, glucose, and lipid profiles were subsequently measured during a refeeding meal test. Prolonged fasting caused a minor reduction of first-phase insulin secretion in a context of improved hepatic insulin action, contrasting an increase in whole body insulin resistance. Accordingly, prolonged fasting was associated with opposite-directed effects on hepatic versus whole body insulin secretion disposition indices. Thirty-six-hour fasting compared with 12-h fasting was associated with increased serum insulin levels during the refeeding meal test. In conclusion, reduced insulin secretion during prolonged fasting may represent a healthy response to improved hepatic insulin action. Use of insulin secretion disposition indices without taking organ-specific insulin action into account may lead to erroneous conclusions.
NEW & NOTEWORTHY Thirty-six-hour prolonged, compared with 12-h overnight fasting, is associated with slightly reduced first-phase insulin secretion in the face of opposite-directed changes in hepatic versus whole body insulin action in healthy young males. The paradoxical finding of increased hepatic versus decreased whole body insulin secretion disposition indices during prolonged fasting challenges the physiological understanding and validity of insulin secretion disposition indices not taking organ-specific insulin action into account.
INTRODUCTION
Many people undergo periodic fasts for health, religious, or cultural reasons, and fasting regimens have been shown to have beneficial effects on inflammation, metabolic stress, and glucose regulation (1–4). Indeed, different periodic fasting strategies, as intermittent and alternate-day fasting, or periods of partial fasting, for example, during the Ramadan, have been shown to revert features of the metabolic syndrome in individuals who are overweight and obese (5–7). However, at the same time, prolonged (>24 h) fasting is associated with markedly decreased peripheral insulin sensitivity (8), lower serum insulin levels [area under the curve (AUC)] 0–10 min after an intravenous glucose bolus, and subsequently with a reduced first-phase insulin secretion relative to whole body insulin sensitivity (i.e., insulin secretion disposition index) (9). Decreased insulin secretion and insulin resistance represents pivotal defects in the pathophysiology of type 2 diabetes, paradoxically contrasting the otherwise putative cardiometabolic health benefits of prolonged fasting.
As for the extent to which decreased insulin secretion during prolonged fasting reflects a true detrimental effect on pancreatic β-cell function, or whether it merely reflects an adaptation to a reduced insulin demand and therefore a potential beneficial “β-cell rest” phenomenon, is currently unknown. Nevertheless, the finding that the reduced insulin secretion during prolonged fasting is observed in a context of reduced insulin action, and thus with a reduced insulin secretion disposition index, appears inconsistent with a beneficial β-cell rest manifestation. One explanation for this paradox, however, could be that fasting-induced insulin resistance is physiologically different from the pathogenic type of insulin resistance predisposing to type 2 diabetes. Skeletal muscle and the liver together account for the two major components of insulin resistance in humans, and insulin resistance in both of these organs is usually present in affluent states in humans including overt type 2 diabetes (10). This, however, may not necessarily be the case during prolonged fasting, at least at the stage where the absolute rate of hepatic glucose production is reduced as shown in humans after 42 h of fasting (11). The putative divergence of hepatic versus peripheral insulin action and resistance during prolonged fasting may represent coordinated compensatory responses to maintain energy delivery to the vital organs heart and brain. Importantly, we previously provided evidence during overfeeding and bedrest of different insulin secretion disposition indices (DIs) when calculated using hepatic versus peripheral insulin resistance, and our data suggested that the pancreatic β-cells more likely sense and adjust insulin secretion to liver as opposed to peripheral (muscle) insulin resistance (12–14). We are unaware of any previous studies reporting on differential effects of prolonged fasting on hepatic versus peripheral insulin resistance, or on hepatic versus peripheral insulin secretion disposition indices.
The aim of the current study was to determine the effects of 36-h (prolonged) versus 12-h (overnight) fasting on hepatic and whole body insulin resistance, as well as on first-phase insulin secretion, and corresponding hepatic versus whole body insulin secretion disposition indices, using stable glucose tracer infusions and a standard intravenous glucose tolerance test (IVGTT), in healthy young men. Serum insulin, plasma glucose, and lipid levels were studied during a refeeding meal test after both 36- and 12-h fasting. We a priori hypothesized that 36- versus 12-h fasting was associated with development of whole body insulin resistance in the face of improved hepatic insulin action. Furthermore, we hypothesized that 36- versus 12-h fasting was associated with a minor reduction of first-phase insulin secretion matching a corresponding minor improvement of hepatic insulin action, and thus with a neutral effect on the hepatic insulin secretion disposition index.
RESEARCH DESIGN AND METHODS
Study Population
We included 13 young and healthy Caucasian men, aged 23–26 yr. All subjects included in this study also participated in our previously published 36-h fasting study (9), which, however, did not include any comparisons of insulin secretion or action, or other metabolic data, after 12-h overnight fasting. Among the 13 participants in the current study, n = 7 were born small for gestational age (SGA), defined as a birthweight <10th percentile, and n = 6 were born appropriate for gestational age (AGA), with birth weights between the 50th–75th percentile of the birth cohort identified through the Danish National Birth Registry. All individuals were born at term (week 39–41). Exclusion criteria were diabetes in the family in two generations, body mass index (BMI) > 30 kg/m2, strenuous exercise >10 h/wk, or use of medication known to affect metabolism. Written informed consent was obtained from all participants. The study was approved by the local ethics committee (H-D-2008-127), and reported investigations were carried out in accordance with the Declaration of Helsinki II.
Study Design
Participants were examined twice with 8–16 wk between studies: 1) during and after a 36-h period of complete fasting (prolonged fasting) and 2) during and after regular feeding and a subsequent 12-h overnight fasting period (overnight fasting; Fig. 1). Both study days were carefully standardized by providing all subjects precooked meals (10 MJ/day) 3 days before the trial (9). The participants had to abstain from alcohol, soft drinks, and exercise during standardization.
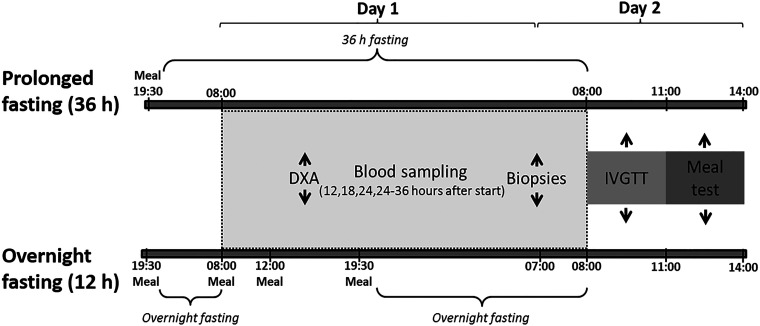
Figure 1.
Study design including overview of study activities and examinations during the prolonged fasting intervention study (36 h) and overnight fasting study. DXA scan, dual-energy X-ray absorptiometry scan; IVGTT, intravenous glucose tolerance test.
Participants arrived at Steno Diabetes Center, Gentofte, at 1930, received a standardized meal to be finished by 2000, and were asked to go to sleep at 2200. Blood sampling began at 0800 in the morning after 36- and 12-h fasting, respectively. Subjects were served three meals and a snack (10 MJ in total) during the overnight fast study. Besides the meals, the two studies were identical (Fig. 1). Ad libitum water was allowed. On day 1, the participants had to spend 2 × 10 min on a stationary bicycle. Weight, height, and waist-hip circumference were measured in the morning. A dual-energy X-ray absorptiometry scan (DXA scan; Hologic Discovery QDR Series) was performed at 1430.
Examinations
Blood sampling (day 1 + 2).
Blood samples were drawn from two antecubital intravenous cannoli at 0800, 1400, and 2000 during day 1 (glucose, insulin/C-peptide, and lipids). From 2000 and onward, samples were drawn every hour for glucose, insulin/C-peptide, and lipids until 0759 at day 2. At 0500, a primed infusion of [6,6-2H2]glucose (4 mg/kg followed by 0.04 mg/kg/min) was administered intravenously, and stable isotopes were measured every 5th min from 0730 to 0759 on day 2.
Intravenous glucose tolerance test (day 2).
As described previously (9), a bolus of 270 mg/kg naturally abundant and 30 mg/kg [6,6-2H2]glucose was administered intravenously as a 20% solution over 2 min starting at 0800.
Blood samples for glucose and insulin/C-peptide were collected at 0, 2, 3, 4, 5, 6, 8, 10, 15, 19, 22, 23, 25, 27, 30, 35, 40, 60, 90, 120, 150, and 180 min. Blood for plasma free fatty acids (FFAs) was collected at 0, 10, 19, 30, and 180 min. Insulin 0.02 IU/kg (Actrapid, Novo Nordisk) was injected intravenously at 0820 to allow for calculation in the minimal model (15).
Meal test (day 2).
A mixed meal test (rye bread sandwiches) was performed after the IVGTT after both 36- and 12-h fasting. The energy content was 39.7E% carbohydrate, 43.6E% fat, and 16.7E% protein in total 2,946 KJ to be ingested within 15 min. Water was allowed. The participants remained in bed for the rest of the trial. Blood samples were drawn at 0, 15, 30, 60, 90, 120, and 180 min for analysis of glucose, insulin/C-peptide, and FFAs, and for triglyceride (TG) concentrations, at 0, 30, 60, 90, 120, and 180 min.
Biochemical Analysis
Blood samples were handled and analyzed as described previously (9). Isotopic analysis of glucose was performed using GC/MS as previous described (16).
Calculations and Statistics
The AUC during day 1, IVGTT, and meal test were calculated using the trapezoid method. The initial β-cell response [first-phase insulin response (FPIR)] was calculated as baseline-adjusted AUC of insulin from 0 to 10 min during the IVGTT. The Phi1 index was calculated as FPIR divided by the baseline-adjusted glucose response from 0 to 10 min during the IVGTT.
A stable glucose isotope was used to calculate rate of appearance (Ra) of glucose [endogenous glucose production (EGP)]. The hepatic insulin resistance index was calculated as the product of EGP multiplied by the serum insulin level at 0600 (Ra glucose × insulin) (17). Glucose tolerance (KG) was determined as the rate constant for glucose clearance after the insulin bolus, expressed as an exponential function. Glucose effectiveness (SG) and the insulin sensitivity index (SI) were calculated from the minimal model using MinMod Millenium v. 6.02 (Richard N. Bergman, Los Angeles, CA) (15). The whole body insulin secretion disposition index (DI) was calculated as (Phi1 × SI), and the hepatic insulin secretion disposition index as (Phi1/Hepatic IR) (17).
Paired Student’s t test and Wilcoxon’s rank-sum test were used to compare paired 36- versus 12-h fasting data, and unpaired Student’s t test and the Mann–Whitney U test were used to compare δ values and unpaired control versus fasting data. Since waking up led to fluctuations in values measured after 0600, analyses were performed on data until this time point except for blood lipids. Data are expressed as means (SE) and means (SD). A P-value of ≤0.05 was considered statistically significant. Analyses were performed using SAS v. 9.4 (SAS Institute, Cary, NC) and GraphPad Prism v. 8.0.2 (GraphPad Software, San Diego, CA).
RESULTS
There were no differences in clinical characteristics of the combined group of 13 AGA and SGA subjects after 12 versus 36 h of fasting (Table 1). The SGA subjects were smaller at birth (P < 0.0001) and had a lower adult height than the AGA subjects (P < 0.05; Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.13181846), corresponding to our findings in a larger cohort (9). Besides this, there were no other differences between the two study subgroups (Supplemental Table S1). Inclusion of both AGA and SGA subjects in the study was done to test our a priori assumption of no major qualitative differences in insulin secretion or action responses to prolonged fasting in SGA subjects with increased risk of type 2 diabetes compared with AGA subjects, which was indeed confirmed. The study was not powered to detect any clinical important quantitative differences of metabolic responses between SGA and AGA subjects, which is the reason why all data included are mean values for the combined group of AGA and SGA subjects. Mean data divided into separate AGA and SGA groups are presented in the online supplement.
Table 1.
Clinical characteristics
| 12-h Fasting | 36-h Fasting | P value | |
|---|---|---|---|
| Weight, kg | 72.9 (8.3) | 73.5 (9.0) | 0.14 |
| BMI, kg/m2 | 22.3 (3.0) | 22.5 (3.1) | 0.25 |
| Waist/hip ratio | 0.91 (0.04) | 0.89 (0.07) | 0.21 |
| Body fat, kg, subtotal | 10.3 (4.5) | 10.7 (4.8) | 0.24 |
| Body fat, %, subtotal | 14.7 (4.4) | 15.3 (4.7) | 0.15 |
Data are means (SD) for 13 individuals. Data were analyzed with Wilcoxon’s signed rank test (paired). BMI, body mass index. Subtotal refers to values being without the head, as several subjects were too tall for the scanner.
Impact of 36- versus 12-h Fasting on Endogenous Glucose Production
The EGP (Ra glucose) was significantly decreased after 36- versus 12-h fasting in all 13 study subjects (P = 0.0007; Table 2). Furthermore, all 13 subjects experienced an improvement of hepatic insulin action with a lower degree of insulin resistance (IR) after 36- versus 12-h fasting (P = 0.0002; Table 2).
Table 2.
Minimal modeling of IVGTT data
| 12-h Fasting | 36-h Fasting | P value | |
|---|---|---|---|
| EGP, Ra glucose mg·min−1 | 138.5 (23.8) | 107.5 (11.0) | 0.0007 |
| EGP, Ra glucose mg·min−1 ·kg−1 | 1.92 (0.37) | 1.47 (0.15) | 0.0007 |
| First-phase insulin response (FPIR) | 1,921 (1,639) | 1,431 (914) | 0.03 |
| First-phase C-peptide response | 8,849 (5,347) | 7,430 (3,766) | 0.13 |
| Phi1 index (FPIR/glucose0–10 min) | 24.70 (16.81) | 18.81 (9.50) | 0.05 |
| Glucose tolerance (KG), min−1 | 1.13 (0.58) | 0.61 (0.05) | 0.002 |
| Glucose effectiveness (SG), h−1 | 0.30 (0.35) | 0.38 (0.24) | 0.33 |
| Whole body insulin sensitivity (SI), pM−1 · h−1 | 0.01 (0.006) | 0.003 (0.004) | 0.002 |
| Hepatic IR, mg/min/kg × ins | 36.8 (11.6) | 12.3 (4.3) | 0.0002 |
| Whole body insulin secretion DI (Phi1 × SI) | 0.22 (0.17) | 0.06 (0.08) | 0.002 |
| Hepatic insulin secretion DI (Phi1/Hep-IR) | 0.67 (0.47) | 1.54 (0.54) | 0.0002 |
Values are means (SD) for 13 individuals. Data were analyzed with Wilcoxon’s signed-rank test (paired). DI, disposition index; EGP, endogenous glucose production; IR, insulin resistance; Ra, rate of appearance. Values in bold indicate statistical significance.
Insulin Secretion and Action Determined by Minimal Modeling during an IVGTT
Absolute first-phase insulin secretion (FPIR) and the baseline adjusted Phi1 index decreased slightly (P = 0.03 and P = 0.05, respectively) after 36- versus 12-h fasting (Table 2). Whole body insulin sensitivity (SI) decreased after 36- versus 12-h fasting (P = 0.002). Glucose tolerance (KG) decreased significantly after 36- versus 12-h fasting (P = 0.002), whereas there was no impact of 36- versus 12-h fasting on glucose effectiveness (SG; Table 2).
Hepatic and Whole Body Insulin Secretion Disposition Indices
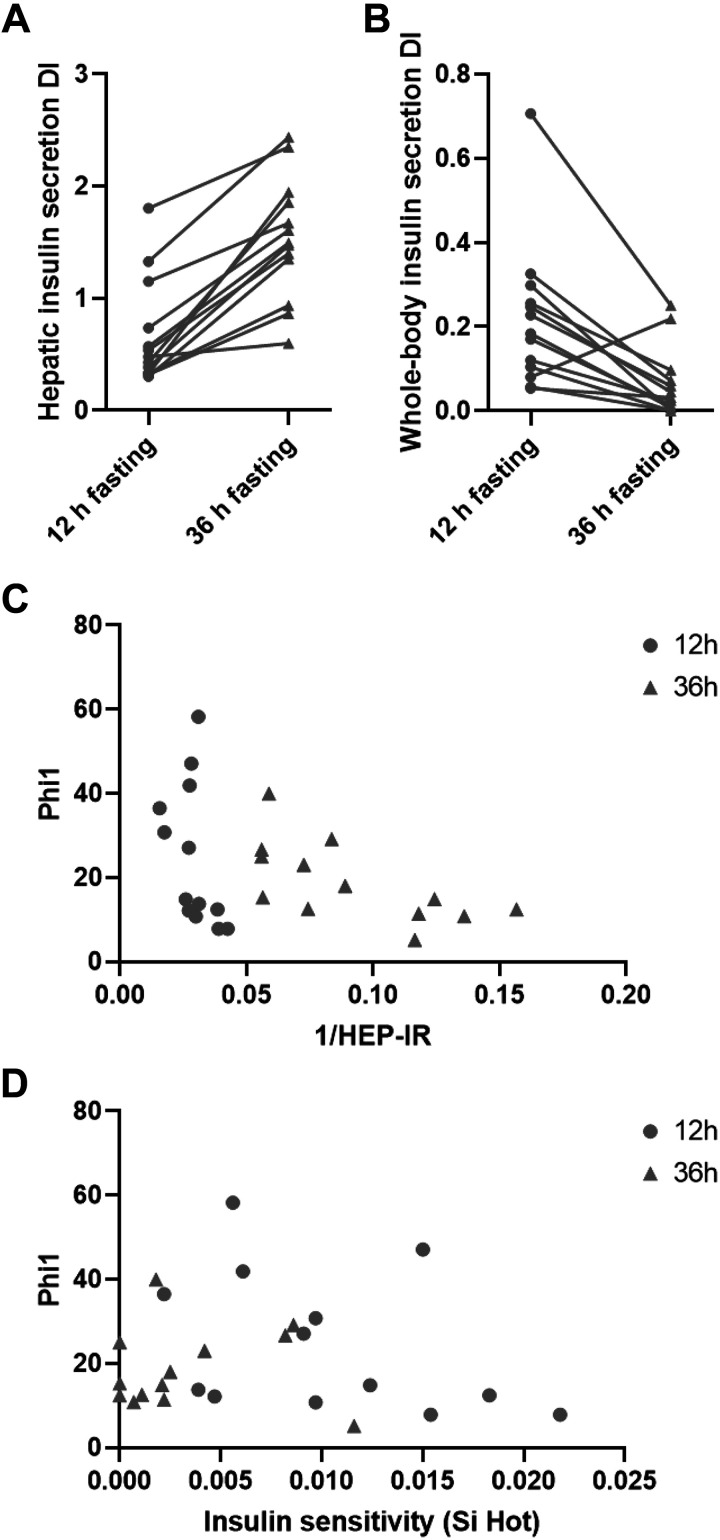
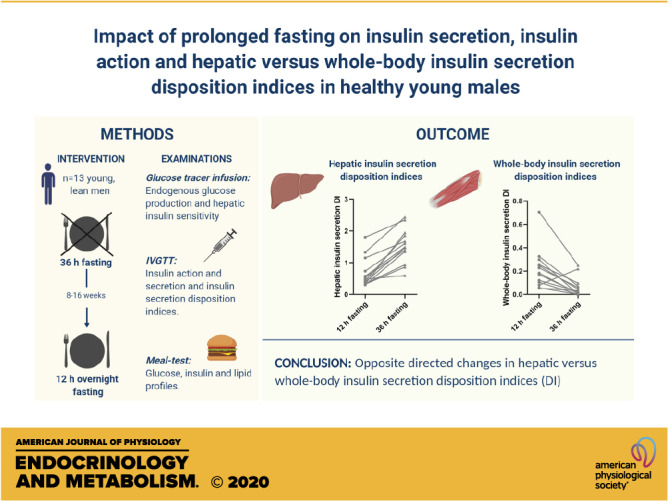
The whole body insulin secretion DI decreased significantly after 36- versus 12-h fasting (P = 0.002). In contrast, the hepatic insulin secretion DI increased significantly (P = 0.0002) after 36- versus 12-h fasting (Table 2). As shown in Fig. 2, the hepatic DI increased for all individuals after 36- versus 12-h fasting (Fig. 2A), whereas the whole body DI decreased for all individuals, except one, after 36- versus 12-h fasting (Fig. 2B). To confirm a hyperbolic relationship, first-phase insulin secretion response was plotted against hepatic insulin sensitivity as well as whole body insulin sensitivity in Fig. 2, C and D.
Figure 2.
Hepatic (A) and whole body (B) insulin secretion DIs for 13 participants after 12-h vs. 36-h fasting. Hyperbolic relationships between first-phase insulin secretion capacity (Phi1) and hepatic insulin action (1/Hep-IR) (C) as well as whole body (peripheral) (D) insulin action (SI) after 12- (circle) and 36-h (triangle) fasting. DIs, disposition indices; SI, insulin sensitivity index.
Glucose, Insulin, and Lipid Profiles Measured during Day 1, IVGTT, and Meal Tests
Day 1.
The AUC for glucose, insulin, C-peptide, and TG levels was significantly decreased after 36- versus 12-h fasting (P range: 0.02–0.0002), whereas the AUC for plasma FFAs increased after 36 versus 12 h (P = 0.002; Table 3).
Table 3.
AUCs during day 1, IVGTT, and meal test in response control (feeding) and fasting
| 12-h Fasting | 36-h Fasting | P value | |
|---|---|---|---|
| Day 1 | |||
| Glucose, mmol/L | 110 (10) | 95 (10) | 0.001 |
| Insulin, pmol/L | 954 (480) | 241 (68) | 0.0002 |
| C-peptide, pmol/L | 15,678 (4013) | 5,318 (1278) | 0.0002 |
| FFAs, µmol/L | 2,675 (740) | 10,120 (2,932) | 0.0002 |
| Triglycerides, mmol/L | 25.5 (4.2) | 20.3 (5.7) | 0.02 |
| IVGTT | |||
| Glucose0–10 min, mmol/L | 129 (25) | 114 (17) | 0.01 |
| GlucoseTotal, mmol/L | 1,039 (116) | 1,178 (111) | 0.002 |
| Insulin0–10min, pmol/L | 2,122 (1,693) | 1,549 (932) | 0.02 |
| InsulinTotal, pmol/L | 11,891 (4,795) | 12,276 (4,160) | 0.64 |
| C-peptide 0–10 min, pmol/L | 13,054 (5,763) | 9,666 (3,982) | 0.002 |
| C-peptideTotal, pmol/L | 103,847 (27,610) | 122,997 (28,333) | 0.03 |
| FFAs, µmol/L | 22,801 (8,944) | 67,935 (16,709) | 0.0002 |
| Meal test | |||
| Glucose, mmol/L | 956 (90) | 1,039 (100) | 0.11 |
| Insulin, pmol/L | 17,063 (6,607) | 22,011 (5,972) | 0.06 |
| C-peptide, pmol/L | 205,880 (58,240) | 294,649 (63,432) | 0.002 |
| FFAs, µmol/L | 306 (113) | 611 (147) | 0.001 |
| Triglycerides, mmol/L | 192 (42) | 155 (39) | 0.02 |
Values are means (SD) for 13 individuals. AUC, area under the curve; FFAs, free fatty acids; IVGTT, intravenous glucose tolerance test. IVGTT0–10 min, AUC of the first 10 min of the IVGTT. IVGTTTotal, AUC of the full 180 min, including the artificial peak from injected insulin. Data were analyzed with Wilcoxon’s signed-rank test (paired). Values in bold indicate statistical significance.
Intravenous glucose tolerance test.
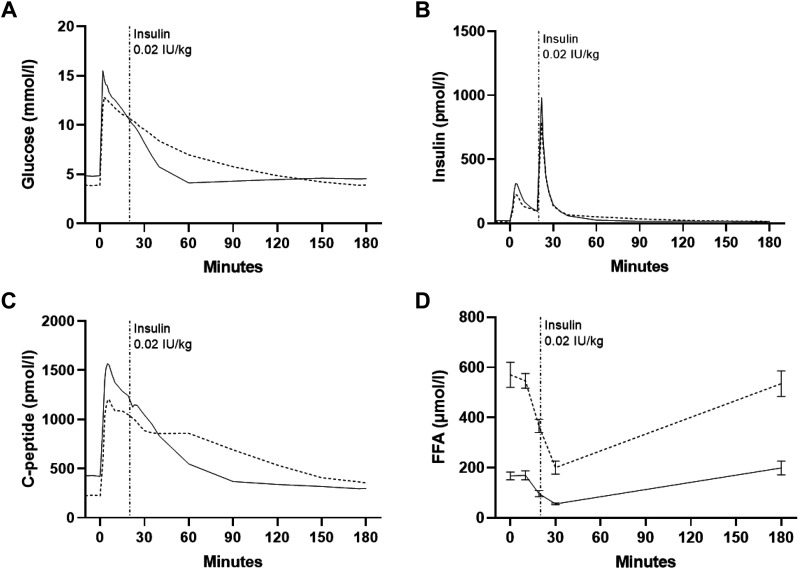
At baseline of the IVGTT (0 min), there was a significant decrease after 36- versus 12-h fasting in plasma glucose (P = 0.002), serum insulin (P = 0.003), and C-peptide (P = 0.0002) concentrations and an increase in plasma FFA concentrations (P = 0.0002; Fig. 3, A–D). AUCs for glucose, insulin, and C-peptide decreased slightly during the initial 0–10 min of the IVGTT after 36- versus 12-h fasting (P range: 0.02–0.002), whereas AUC for the total duration of the IVGTT was significantly increased for glucose, C-peptide, and FFAs (P range: 0.03–0.0002; Table 3).
Figure 3.
Plasma glucose (A), serum insulin (B), serum C-peptide (C), and plasma free fatty acids (FFAs) (D) curves during the frequently sampled intravenous glucose tolerance test (IVGTT). Solid lines, 12-h fasting; dotted lines, 36-h fasting. Data are means ± SE (FFAs only) for 13 participants. SE has been removed due to the high number of sampling points.
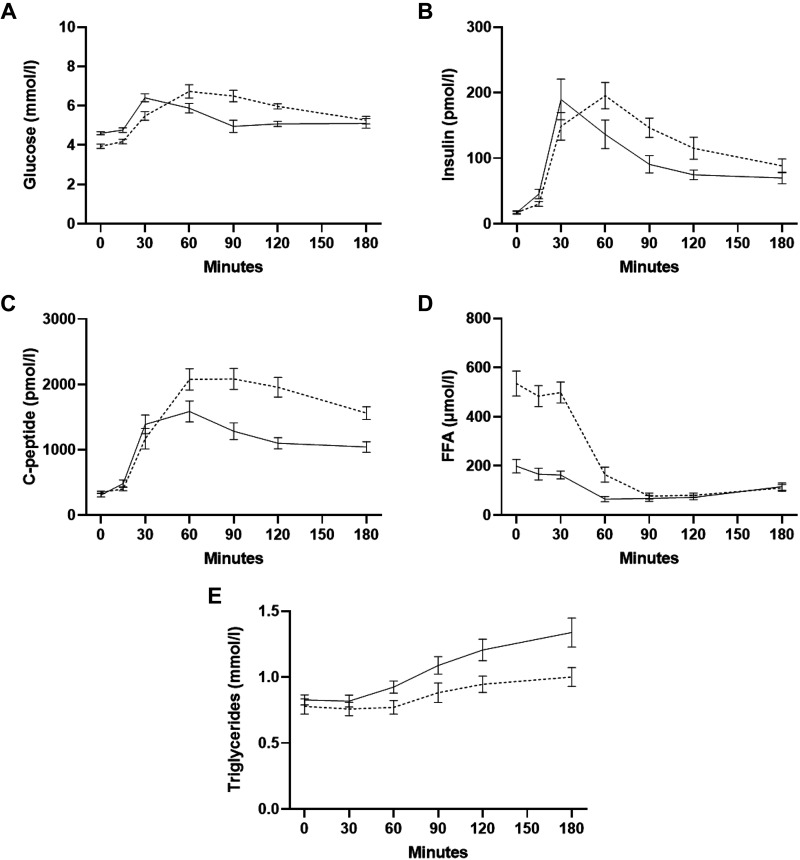
Meal test.
At baseline of the meal test (0 min), plasma glucose was significantly lower (P = 0.0005) and C-peptide and FFA concentrations significantly increased (P = 0.04 and P = 0.0002) after 36- versus 12-h fasting, with no differences in serum C-peptide or plasma TG concentrations (Fig. 4, A–E). The AUC for serum C-peptide and plasma FFAs was significantly increased (P = 0.002 and P = 0.001), and the AUC for plasma TG significantly decreased (P = 0.02) after 36- versus 12-h fasting (Table 3).
Figure 4.
Plasma glucose (A), serum insulin (B), serum C-peptide (C), plasma free fatty acids (FFAs) (D), and plasma triglycerides (TG) (E) curves during the meal test. Solid lines, 12-h fasting; dotted lines, 36-h fasting. Data are means ± SE for 13 participants.
Differential metabolic responses to prolonged fasting in SGA versus AGA subjects.
EGP declined slightly more in the SGA subgroup compared with the AGA subgroup after 36- versus 12-h fasting (δ EGP: P = 0.045; Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.13181897). Furthermore, plasma TG levels at baseline, i.e., before the meal test, as well as the AUC for TG, were significantly lower (P = 0.015 and P = 0.03) after 36- versus 12-h fasting in the SGA group only (Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.13182017; and Supplemental Fig. 3E; see https://doi.org/10.6084/m9.figshare.13182317). All other metabolic characteristics and responses to prolonged fasting were similar in AGA versus SGA subjects (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.13182293.v1 and Fig. S2; see https://doi.org/10.6084/m9.figshare.13182314.v1).
DISCUSSION
Prolonged (36 h) versus overnight (12 h) fasting in young healthy men was associated with lower fasting plasma glucose and insulin levels, as well as with markedly lower rates of hepatic glucose production and of hepatic insulin resistance. In contrast, whole body insulin sensitivity, primarily reflecting peripheral tissues, decreased markedly in all subjects after prolonged versus overnight fasting. Absolute first-phase insulin secretion (Phi1) decreased marginally following 36-h fasting. Subsequently, 36- versus 12-h fasting was associated with significant and opposite-directed paradoxical changes in hepatic versus whole body insulin resistance-adjusted insulin secretion disposition indices (DIs; Fig. 2, A and B).
Whole body insulin action and resistance represent the net product of insulins capability to suppress endogenous (hepatic) glucose production on one side and to enhance glucose uptake into peripheral tissues, primarily muscle, on the other side. Hepatic glucose production is rapidly suppressed during the initial phase of the IVGTT (18), and previous studies showed that the IVGTT-calculated whole body insulin sensitivity index predominantly mirrors insulins effect on peripheral glucose uptake (19, 20). Indeed, our finding of whole body insulin resistance during prolonged fasting using the IVGTT is consistent with previous studies showing peripheral insulin resistance during fasting as determined by the gold-standard euglycemic-hyperinsulinemic clamp technique (21, 22). In contrast, we here for the first time have documented increased hepatic insulin action at low basal insulin levels in healthy young subjects after prolonged compared with overnight fasting (Table 2). Our finding of a reduced absolute rate of glucose appearance after prolonged fasting is consistent with a previous 42-h fasting study (23).
Salgin et al. (24) previously reported decreased first-phase insulin secretion during a standard IVGTT after 24-h versus overnight fasting in healthy subjects. In accordance with Salgin et al., we found marginally reduced first-phase (0–10 min) insulin secretion (FPIR) during the IVGTT after prolonged versus overnight fasting (Table 2). This was irrespective of whether first-phase insulin secretion was expressed simply as serum insulin areas under the curve (AUC) or as the Phi1 index, taking into account the reduced 0–10-min plasma glucose AUC after prolonged versus overnight fasting in both groups (Table 3). Also, in agreement with Salgin et al., we found reduced insulin secretion after prolonged versus overnight fasting when adjusting Phi1 with whole body insulin resistance (whole body DI; Table 2). Nevertheless, we previously provided data and arguments for pancreatic insulin secretion being more likely to sense and respond to changes in hepatic as opposed to peripheral insulin resistance (12–14). In agreement with our a priori hypothesis, prolonged versus overnight fasting markedly improved hepatic insulin action in all study subjects. Interestingly, and in contrast to our a priori hypothesis, the improvement of hepatic insulin sensitivity far exceeded the decline in absolute first-phase insulin secretion, resulting in that first-phase insulin secretion to intravenous glucose adjusted for hepatic insulin action, namely, the hepatic insulin secretion DI was not only neutral but actually increased markedly during prolonged fasting (Table 2). Accordingly, our results support the notion that prolonged fasting induces a normal physiological adaptation of the pancreatic insulin secretion to improved hepatic insulin action, providing insulin sparing and β-cell rest by allowing insulin to accumulate in the pancreatic β-cell granule. Indeed, the first-phase insulin response is thought to represent release of preformed insulin, whereas the second-phase release from 10 min and onward represent newly produced insulin (25, 26). As for the lower KG value during IVGTT after prolonged compared with short-term overnight fasting, this is most likely to be explained primarily by the severe peripheral insulin resistance as opposed to the slightly lower absolute first-phase insulin secretion.
The nutritional state of an individual is worth considering in the discussion of the relevance of adjusting insulin secretion for hepatic or peripheral insulin resistance. Indeed, the much lower serum insulin levels during prolonged fasting reflects a state where the liver plays a dominant role in glucose homeostasis, and it may be speculated that the β-cell during low insulin levels adapt primarily to hepatic as opposed to peripheral insulin action. Conversely, in metabolically more affluent high insulin and postprandial states, including severe obesity, it is likely that the β-cells adapt relatively more to peripheral glucose homeostasis and insulin action. The specific signal from insulin responsive organs to the β-cells of a need to adjust insulin secretion, justifying the use of any insulin secretion disposition index, is not known. However, the most likely mediating signal remains subtle changes in plasma glucose or a related metabolite level. Indeed, if this is the case, it supports the notion that any correction of β-cell function to insulin action should be performed taking into account which organ(s), liver and/or periphery (muscle), has played the primary role in controlling whole body glucose homeostasis during the time of testing.
A prerequisite for the use of insulin secretion disposition indices is the consistent finding of a hyperbolic inverse relationship between insulin secretion and insulin action (11, 27–29). We have previously documented inverse hyperbolic relationships between both peripheral and hepatic insulin secretion and action measurements in healthy and prediabetic groups (12–14). Indeed, we here once again can document inverse hyperbolic relationships between insulin secretion and estimates of both hepatic as well as whole body insulin resistance, as determined after both overnight and prolonged fasting (Fig. 2, C and D). If anything, the relationship between insulin secretion and hepatic insulin action before and after prolonged fasting may fit the inverse hyperbolic relationship better than the relationship between insulin secretion and whole body insulin action (Fig. 2, C and D). However, it is important to note that the minor change in absolute first-phase insulin secretion was neither a perfect match [e.g., defined as same (constant) DIs in the two different study conditions] to the change (improvement) in hepatic action, nor to the change (deterioration) in whole body insulin resistance/action. This may be explained by the timing and/or dynamics of the adaptation, with none of the three functions (insulin secretion, hepatic insulin action, or peripheral insulin action) having reached steady state. Other explanations include the possibility that the role of peripheral insulin action in the adaptation of β-cell function is not completely offset during the experimental fasting conditions. In other words, the adjustment of insulin secretion might be a compromise between the opposite-directed changes in hepatic versus peripheral distinct organ insulin sensitivities. Finally, an equally plausible explanation for this may be that some (or potentially none) of the otherwise considered gold-standard measurements of in vivo insulin secretion or insulin action used in this study fully capture the complex organ physiology including not the least the adjustment of insulin secretion to organ in vivo insulin action. Regardless, the current data question the validity of conventional insulin disposition indices, not taking differential organ insulin action into account. Indeed, there is an urgent need for studies addressing cross talk between different insulin-sensitive organs on one side and physiological adjustments of insulin secretion on the other side.
As for the extent to which prolonged fasting is beneficial or detrimental to the insulin secretion machinery, the serum insulin and C-peptide profiles during the refeeding meal test do to some extent support a beneficial β-cell rest effect. Thus, in contrast to the marginally lower serum insulin and C-peptide levels during the IVGTT after prolonged fasting, serum insulin and C-peptide levels were significantly increased during the (compared with IVGTT) more energy-rich and physiological refeeding meal, which also includes β-cell stimulation with other nutrients such as amino acids and incretin hormones after 36- versus 12-h fasting (Table 3; Figs. 3 and 4). This in face of near similar plasma glucose levels during the two study conditions.
It was shown previously that the elevated basal hepatic glucose production in hyperinsulinemic type 2 diabetic patients is closely associated with a lower responsiveness to the suppression of the rate of hepatic glucose production during experimental exogenous (clamp) low-grade physiologic hyperinsulinemia, providing some validation of the hepatic insulin sensitivity index as determined after an overnight fast (30). Indeed, multiple studies have used the hepatic insulin sensitivity index as a prime measure of hepatic insulin sensitivity (31–34). However, there is a need to validate and understand the performance of the hepatic insulin sensitivity index versus exogenous insulin infusion clamp determinations of hepatic insulin action after prolonged fasting when the liver glycogen levels have been depleted (35). The net hepatic glucose production rate is the sum of the rate of glycogenolysis and gluconeogenesis, which to some unknown extent may influence the dose responsiveness of insulin to suppress hepatic glucose production after prolonged fasting. Accordingly, the hyperbolic relationship between first-phase insulin secretion and the hepatic insulin sensitivity index after prolonged fasting, as seen in this study, may not necessarily be the same with hepatic insulin sensitivity measures obtained from exogenous insulin infusion clamp studies. A previous study reported concordant improvements of both first-phase insulin secretion as well as exogeneous insulin infusion clamp determined hepatic insulin action after 1 wk of severe calorie restriction in type 2 diabetic patients (36). Besides supporting our results of improved hepatic insulin action after prolonged fasting as determined by using the hepatic insulin sensitivity index, this study in addition provides some support for our conclusion of fasting (and/or calorie restriction) being associated with a relative or absolute improvement of insulin secretion, rather than an impairment of insulin secretion as reported by others (24).
We previously reported that SGA subjects with increased risk of developing type 2 diabetes may benefit preferentially from 36-h fasting with respect to lowering fasting serum insulin and triglyceride (TG) levels compared with AGA subjects (9). To ensure the results of the current study being qualitatively relevant to a broader range of people with normal glucose tolerance, we included both SGA and AGA subjects in this study. Indeed, all individual SGA and AGA study subjects improved the hepatic insulin secretion index, whereas all except one subject conversely showed a deterioration of the whole body insulin secretion index in response to prolonged versus overnight fasting (Fig. 2, A and B). With the low number of subjects in each of the two subgroups, however, we of course cannot exclude minor quantitative differences in responses of insulin secretion indices to prolonged fasting between SGA and AGA subjects. The finding of relatively less improvement of hepatic insulin action during prolonged fasting in SGA compared with AGA subjects is explained entirely by a nonsignificantly lower hepatic insulin action after overnight fasting in the SGA subjects and may therefore represent an incidental finding. The finding of reduced fasting and refeeding (meal test) post prandial plasma TG levels in response to prolonged fasting only among SGA subjects (Supplemental Table S3 and Supplemental Fig. S3) is in line with our previous prolonged fasting study in a larger group of SGA and AGA subjects (9).
CONCLUSIONS
Prolonged compared with overnight fasting is associated with opposite-directed changes in hepatic versus whole body insulin action and resistance in healthy young males. We hypothesize that the apparent minor reductions in circulating serum insulin and C-peptide levels during prolonged fasting primarily reflects adjustments to improvements of hepatic insulin action. The documented opposite-directed differences in whole body versus hepatic insulin secretion disposition indices in response to prolonged fasting may question our physiological understanding of the insulin secretion disposition indices, not taking organ-specific insulin action into account. Further studies are urgently needed.
GRANTS
The study was funded by the European Foundation for the Study of Diabetes (EFSD), The Danish Council for Strategic Research, Novo Nordisk Fonden, The Danish Diabetes Academy supported by the Novo Nordisk Foundation, The Augustinus Foundation, and The European Union 6th Framework EXGENESIS grant.
DISCLOSURES
C. Brøns is a shareholder in Novo Nordisk A/S, and A. A. Vaag is a shareholder in AstraZeneca. No other authors report conflicting interest.
AUTHOR CONTRIBUTIONS
S.W.J., C.B., and A.A.V. conceived and designed research; S.W.J. and L.H. performed experiments; S.W.J., L.H., L.G., L.J., and C.B. analyzed data; S.W.J., L.H., L.G., L.J., S.M., C.B., and A.A.V. interpreted results of experiments; L.J. prepared figures; C.B. and A.A.V. drafted manuscript; S.W.J., L.H., L.G., L.J., S.M., C.B., and A.A.V. edited and revised manuscript; S.W.J., L.H., L.G., L.J., S.M., C.B., and A.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Modest and L. S. Koch of the Steno Diabetes Center, Gentofte, for technical support and laboratory assistance. We are especially grateful to all the young men who participated in this study.
REFERENCES
- 1.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381: 2541–2551, 2019. [Erratum in N Engl J Med 382: 298, 2020]. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 2.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab 19: 181–192, 2014. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maughan RJ, Fallah J, Coyle EF. The effects of fasting on metabolism and performance. Br J Sports Med 44: 490–494, 2010. doi: 10.1136/bjsm.2010.072181. [DOI] [PubMed] [Google Scholar]
- 4.Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, Martínez ME, Villaseñor A, Sears DD, Marinac CR, Gallo LC. Intermittent fasting and human metabolic health. J Acad Nutr Diet 115: 1203–1212, 2015. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35: 714–727, 2011. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 81: 69–73, 2005. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 90: 1138–1143, 2009. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 8.Björkman O, Eriksson LS. Influence of a 60-hour fast on insulin-mediated splanchnic and peripheral glucose metabolism in humans. J Clin Invest 76: 87–92, 1985. doi: 10.1172/JCI111982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen SW, Brøns C, Bluck L, Hjort L, Færch K, Thankamony A, Gillberg L, Friedrichsen M, Dunger DB, Vaag AA. Metabolic response to 36 hours of fasting in young men born small vs appropriate for gestational age. Diabetologia 58: 178–187, 2015. [Erratum in Diabetologia 58: 204, 2015]. doi: 10.1007/s00125-014-3406-6. [DOI] [PubMed] [Google Scholar]
- 10.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98: 2133–2223, 2018. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP , et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993. doi: 10.2337/diabetes.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 12.Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Stallknecht B, Dela F, Vaag A. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 58: 2749–2756, 2009. doi: 10.2337/db09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, Jacobsen S, Nilsson E, Larsen CM, Astrup A, Quistorff B, Vaag A. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587: 2387–2397, 2009. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Færch K, Brøns C, Alibegovic AC, Vaag A. The disposition index: adjustment for peripheral vs. hepatic insulin sensitivity? J Physiol 588: 759.–, 2010. doi: 10.1113/jphysiol.2009.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson SJ, Waterhouse JS, Bluck LJ. A single glucose derivative suitable for gas chromatography/mass spectrometry and gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom 21: 3123–3128, 2007. doi: 10.1002/rcm.3195. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30: 89–94, 2007. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 18.Krudys KM, Dodds MG, Nissen SM, Vicini P. Integrated model of hepatic and peripheral glucose regulation for estimation of endogenous glucose production during the hot IVGTT. Am J Physiol Endocrinol Metab 288: E1038–E1046, 2005. doi: 10.1152/ajpendo.00058.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 79: 790–800, 1987. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksen JE, Alford F, Handberg A, Vaag A, Ward GM, Kalfas A, Beck- Nielsen H. Increased glucose effectiveness in normoglycemic but insulin-resistant relatives of patients with non-insulin-dependent diabetes mellitus. A novel compensatory mechanism. J Clin Invest 94: 1196–1204, 1994. doi: 10.1172/JCI117436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeks J, van Herpen NA, Mensink M, Moonen-Kornips E, van Beurden D, Hesselink MK, Schrauwen P. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes 59: 2117–2125, 2010. doi: 10.2337/db10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Crabben SN, Allick G, Ackermans MT, Endert E, Romijn JA, Sauerwein HP. Prolonged fasting induces peripheral insulin resistance, which is not ameliorated by high-dose salicylate. J Clin Endocrinol Metab 93: 638–641, 2008. doi: 10.1210/jc.2006-2491. [DOI] [PubMed] [Google Scholar]
- 23.de Zegher F, Ong K, van Helvoirt M, Mohn A, Woods K, Dunger D. High-dose growth hormone (GH) treatment in non-GH-deficient children born small for gestational age induces growth responses related to pretreatment GH secretion and associated with a reversible decrease in insulin sensitivity. J Clin Endocrinol Metab 87: 148–148, 2002. doi: 10.1210/jcem.87.1.8293. [DOI] [PubMed] [Google Scholar]
- 24.Salgin B, Marcovecchio ML, Humphreys SM, Hill N, Chassin LJ, Lunn DJ, Hovorka R, Dunger DB. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am J Physiol Endocrinol Metab 296: E454–E461, 2009. doi: 10.1152/ajpendo.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52: 739–751, 2009., doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 26.Rorsman P, Eliasson L, Renström E, Gromada J, Barg S, Göpel S. The cell physiology of biphasic insulin secretion. News Physiol Sci 15: 72–77, 2000. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- 27.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 56: 1703–1711, 2007. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- 28.Cobelli C, Toffolo GM, Man CD, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of β-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293: E1–E15, 2007. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the san antonio metabolism study. Diabetes 66: 815–822, 2017. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 30.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213, 1989. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choukem S-P, Gautier J-F. How to measure hepatic insulin resistance? Diabetes Metab 34: 664–673, 2008. doi: 10.1016/S1262-3636(08)74602-0. [DOI] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, DeFronzo RA. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133: 496–506, 2007. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 34.Tripathy D, Merovci A, Basu R, Abdul-Ghani M, DeFronzo RA. Mild physiologic hyperglycemia induces hepatic insulin resistance in healthy normal glucose-tolerant participants. J Clin Endocrinol Metab 104: 2842–2850, 2019. doi: 10.1210/jc.2018-02304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254: 573–576, 1991. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 36.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54: 2506–2514, 2011. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]