Keywords: glucose tolerance, intermittent hypoxia, sex hormones, sex-specific, sleep apnea
Abstract
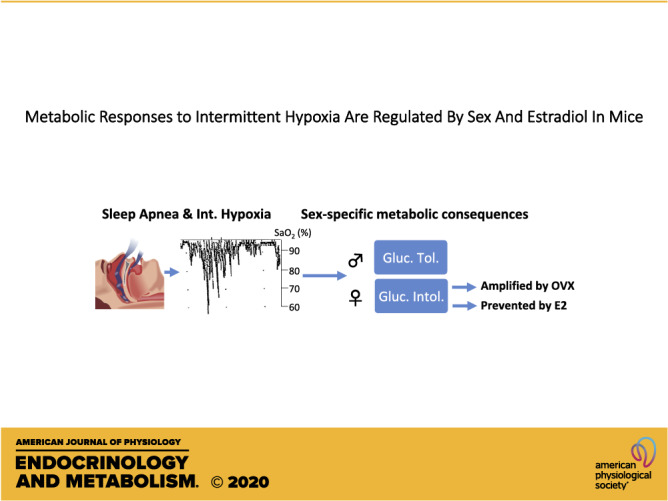
The roles of sex and sex-hormones on the metabolic consequences of intermittent hypoxia (IH, a reliable model of sleep apnea) are unknown. We used intact male or female mice and ovariectomized (OVX) females treated with vehicle (Veh) or estradiol (E2) and exposed to normoxia (Nx) or IH (6% O2, 10 cycles/h, 12 h/day, 2 wk). Mice were then fasted for 6 h, and we measured fasting glucose and insulin levels and performed insulin or glucose tolerance tests (ITT or GTT). We also assessed liver concentrations of glycogen, triglycerides (TGs), and expression levels of genes involved in aerobic or anaerobic metabolism. In males, IH lowered fasting levels of glucose and insulin, slightly improved glucose tolerance, but altered glucose tolerance in females. In OVX-Veh females, IH reduced fasting glucose and insulin levels and strongly impaired glucose tolerance. E2 supplementation reversed these effects and improved homeostasis model assessment of β-cell function (HOMA-β), a marker of pancreatic glucose-induced insulin released. IH decreased liver TG concentration in males and slightly increased glycogen in OVX-Veh females. Liver expression of glycolytic (Ldha) and mitochondrial (citrate synthase, Pdha1) genes was reduced by IH in males and in OVX-Veh females, but not in intact or OVX-E2 females. We conclude that 1) IH reduced fasting levels of glycemia in males and in ovariectomized females. 2) IH improves glucose tolerance only in males. 3) In females IH decreased glucose tolerance, this effect was amplified by ovariectomy, and reversed by E2 supplementation. 4) During IH exposures, E2 supplementation appears to improve pancreatic β cells functions.
NEW & NOTEWORTHY We assessed fasting glycemic control, and tolerance to insulin and glucose in male and female mice exposed to intermittent hypoxia. IH improves glucose tolerance in males but had opposite effects in females. This response was amplified following ovariectomy in females and prevented by estradiol supplementation. Metabolic consequences of IH differ between males and females and are regulated by estradiol in female mice.
INTRODUCTION
Although the higher prevalence of sleep apnea in men than in women has been established >40 years ago (1), and regularly confirmed since then (2), the fact that sleep apnea has sex-specific consequences has recently emerged as an important feature of this respiratory disease (3–6). Metabolic disturbances are common in patients with sleep apnea, and a large cross-sectional study, performed on a general population with low prevalence of obesity, showed that the severity of sleep apnea is associated with diabetes in men but not in women, whereas an association with metabolic syndrome appeared after menopause in women (5). Similar sex-specific comorbidities of sleep apnea have been reported in a nationwide survey in the United States, showing higher prevalence of type 2 diabetes in men with apnea versus women with apnea (6).
The mechanisms underlying these abovementioned differences remain poorly understood, and so far, there are no experimental studies available to help understand the interactions between sex or sex-hormones and the metabolic consequences of sleep apnea. In rodents, exposure to intermittent hypoxia (IH) is an efficient tool to study the consequences of sleep apnea, and it induces profound alterations of adipose tissue (7, 8), pancreatic β cells (9), liver (10), or skeletal muscle functions (11), contributing to metabolic dysregulations. We, therefore, tested the hypotheses that IH exposures induce sex-specific metabolic consequences in mice, and that circulating estradiol (E2) in females explains these differences.
MATERIALS AND METHODS
Ethical Approval
All experiments have been approved by the Animal Protection Committee of Université Laval in accordance with the Canadian Council on Animal Care in Science. We used a total of 20 male and 75 female mice (2–3 months old). All were C57BL/6NCrl mice procured from Charles-River Laboratories (Saint-Constant, QC, Canada). All mice had ad-libitum access to food and water and were maintained on a 12/12-h light/dark cycle.
General Experimental Design
Two weeks after their arrival in our animal house facility, male mice were left intact and females were either left intact or ovariectomized (OVX). During the surgery, OVX females were implanted with an osmotic minipump for continuous delivery of vehicle (Veh) or E2 (0.5 µg/day, see Ovariectomy and implantation of osmotic pumps for details) for up to 4 wk. Two weeks following the surgery and pump implantation, the mice were exposed to IH (10 cycles/h, nadir 6% O2, 12 h/day, for 14 consecutive days) or maintained in normoxia (Nx). Following the last day of exposure, mice were fasted for 6 h (0700–1300), and then used either for an intraperitoneal insulin tolerance test (ITT, ip insulin injection, 0.65 U/kg), a glucose tolerance test (GTT, ip glucose injection, 2 g/kg), or for liver sampling.
Experimental Details
Ovariectomy and implantation of osmotic pumps.
Mice were anesthetized with isoflurane (4% induction, 2% thereafter, in 30% O2), and the level of anesthesia was assessed by the lack of reflex responses to tail pinch. A local analgesia was applied before the surgery (lidocaine + bupivacaine, subcutaneous injection, respectively, 7 and 3.5 mg/kg). The ovaries were removed, and an osmotic pump was implanted in the upper mid-back region (Alzet; model 1004, flow of 0.11 μL/h for 28 days). Each pump delivered vehicle (Veh: 50% DMSO in propylene glycol, respectively, from Bioshop Canada, Burlington, ON, and J.T. Baker) or estradiol (E2—0.5 µg/day—Sigma Aldrich; this dosage leads to E2 plasma level in the range of intact females in proestrus (12, 13). All mice received pre- and postoperative analgesics for 48 h (buprenorphin and meloxican, subcutaneous injection, respectively, 0.01 and 5 mg/kg), according to our normalized procedures. Two weeks later, the mice were exposed to either Nx or IH for 14 consecutive days.
Intermittent hypoxia exposure.
Mice were housed in standard cages (5 mice/cage) connected to an oxycycler (Biospherix, Redfield, NY). Oxygen dropped from 21% to 6% in 90 s, remained at 6% for 30 s, and then returned to 21% in 70 s, holding normoxia for 170 s. There were 10 cycles/h for 12 consecutive hours between 0600 AM and 1800 PM for 14 consecutive days.
Insulin tolerance test.
After 14 days of exposure to Nx or IH, mice were fasted for 6 h starting at 0700 AM, and then used to perform an insulin tolerance test (ITT) by intraperitoneal injection of insulin (0.65 U/kg, Humulin R). The day before the test, the right leg of the mice was shaved for easy access to the lateral saphenous vein. On the day of the test, a first blood sample was drawn (∼100 µL by puncture of the vein, t0 = baseline) and the plasma was immediately frozen. Mice were then placed within restrainer tubes for the duration of the test and received the intraperitoneal insulin injection. Subsequent blood samples were drawn by puncture of the vein at 15, 30, 60, and 90 min following insulin injection. Blood glucose levels were measured by a portable glucometer (Accu-Chek, Aviva) during the ITT. Blood samples at t0 (baseline) were later used for assays of fasting levels of insulin using a standard assay kit (Alpco Diagnostics, Salem, NH).
GTT.
GTT were performed on another group of mice exposed to similar conditions of Nx or IH for 2 wk and 6 h of fasting. Mice were prepared as described in Insulin tolerance test. A first blood sample was drawn (∼100 µL by puncture of the vein, t0 = baseline) and the plasma was immediately frozen. Mice were then placed within restrainer tubes for the duration of the test and received an intraperitoneal injection of glucose (2 g/kg). Subsequent blood samples were drawn by puncture of the saphenous vein at 5, 10, 15, 30, 60, and 90 min, and glucose levels were measured by the glucometer. Because the circulating glucose levels in intact males (in Nx) and OVX females (in IH) quickly exceeded the reading capacity of the portable device (>32 mM), samples at t15 were used to measure the glucose levels in these groups by a standard biochemical assay (Crystal Chem. No. 81692). Blood samples at t0 were later used for assays of fasting levels of insulin using a standard assay kit (Alpco Diagnostics).
HOMA-IR, HOMA-β, area under the curve for ITT and GTT.
The homeostasis model assessment of insulin resistance (HOMA-IR) and pancreatic β cell functions (HOMA-β) were calculated using glucose and insulin concentrations measured in samples of blood that have been drawn at t0 during the ITT and GTT. HOMA-IR was calculated as [insulin] × [glucose]/22.5, and HOMA-β as [insulin]/[glucose] (14–16). For the ITT and GTT, the area under the curve (AUC) were calculated for each animal either using the absolute values of glucose concentrations (GTT) or normalized to t0 levels (ITT).
Hormone assays.
Plasma samples taken at baseline during the ITT and GTT were used to measure the concentrations of E2 (ELISA kit No. 501890, Cayman Chemical, Ann Arbor, MI) and corticosterone (ELISA kit No. ADI-900-097, Enzo Life Sciences, Burlington, ON, Canada). Assays were conducted according to the manufacturer’s instructions.
Dissection of liver tissues.
Liver tissues were dissected from other groups of mice exposed to similar conditions of Nx or IH for 2 wk and 6 h of fasting. They were used to measure the tissue concentrations of glycogen and triglyceride (TG), and the mRNA expression levels of key genes involved in aerobic (Pdha1: pyruvate dehydrogenase α1 subunit, which contains the active site of the PDH complex; Pdk1: pyruvate dehydrogenase kinase 1, which inhibit PDH activity by phosphorylation of PDHα1; Cs: citrate synthase) or anaerobic (Ldha: lactate dehydrogenase A) glucose metabolism.
Extraction and measurement of liver glycogen concentrations.
As described previously (17, 18), glycogen was extracted from 30–50 mg liver samples in potassium hydroxide (KOH) 30% saturated with Na2SO4, incubated 30 min at 70°C, and cooled on ice, then precipitated in 95% ethanol and centrifuged at 840 g. The pellet was resuspended in 3 mL distilled water. Concentration was calculated using a standard curve made from commercial glycogen (Cat. No. 10901393001 Roche). 50–250 µL of sample was prepared in triplicate, mixed with 5% phenol and 96%–98% H2SO4, incubated for 20 min at 30°C and absorbance read at 490 nm.
Extraction and measurement of liver TG concentrations.
TG from liver samples (around 50 mg) were extracted as described before (17). Briefly, liver samples were homogenized in 900 μL of chloroform:methanol 2:1. The homogenate was combined with 300 μL methanol, vortexed, and centrifuged for 15 min at 3,000 rpm. Supernatant of 825 μL was transferred to a glass tube, added with 400 μL chloroform and 275 μL NaCl 0.73%, vortexed for 30 s and centrifuged at 5,000 rpm for 3 min. The upper phase was discarded; 800 μL of chloroform:methanol:NaCl 0.58% (3:48:47) was added to wash the lower phase and centrifuged at 5,000 rpm for 3 min. After three washes, the lower phase was evaporated and resuspended in 1 mL of fresh isopropanol. TG levels were determined using a standard assay kit (Thermo Fisher Scientific, TR22421) according to the manufacturer’s instructions.
Extraction of RNA, cDNA synthesis, and quantitative real time qPCR.
Total RNA was extracted from snap-frozen liver samples using EasyPure RNA Kit (TransGen Biotech Co. Cat. No. ER 101, China) according to the manufacturer’s instructions. Briefly, frozen samples were weighed and mechanically homogenized in binding buffer (300 µL/10 mg), a proteinase K solution (15 µL/10 mg), and β-mercaptoethanol (1%), incubated for 15 min at 56°C and centrifuged at 12,000 g for 5 min at room temperature. Then RNA was purified by adding an equal volume of 70% ethanol to the lysate and vortexed. It was then centrifuged for 20 s at 12,000 g, the lysate was added into the spin columns, and recentrifuged for 30 s at 12,000 g. The columns were then cleaned (500 µL of clean buffer, centrifuged at 12,000 g for 35 s), the DNA digestion was performed by adding 80 µL of DNAse I for 15 min, cleaned again, then washed twice (500 µL of washing buffer, centrifuged at 12,000 g for 35 s), followed by a centrifugation at maximum speed of 2 min. The column was then air-dried for 3 min and RNA was eluted with water (100 µL). All tubes were transferred on ice and total RNA concentration was determined by the absorption at 260 nm using a Thermo Scientific NanoDrop 2000c spectrophotometer (Fisher Scientific, MA). The 260/280 nm and 230/260 nm absorption ratio were assessed. All samples had absorption ratios of 260/280 > 1.8 and 260/230 typically >1.3. RNAs were then stored at −80°C until further use.
For cDNA synthesis, 1,000 ng of RNA was reverse transcribed using the iScriptTM Advanced cDNA Synthesis Kit for RT-qPCR (Bio-Rad Laboratories, Mississauga, ON, Canada) following the manufacturer’s instructions. Briefly, cDNA synthesis reaction was prepared by mixing 4 µL reaction mix, 1 µL reverse transcriptase, 1,000 ng RNA, and nuclease free water (final volume 20 µL), and then placed for 20 min at 46°C, followed by 1 min at 95°C. The cDNA was then stored at −80°C until further use.
The sequences of primer pairs used are listed in Table 1. RT-qPCR amplifications were performed using a SYBR Green Core Kit on a CFX384 Real-Time System C1000 Touch Thermal Cycler (Bio-Rad Laboratories, Mississauga, ON, Canada). Initial amplification was done with a denaturation step at 95°C for 10 min followed by 45 cycles of denaturation at 95°C for 10 s, primer annealing at 56°C–60°C (depending on the primer) for 15 s, and primer extension at 72°C for 20 s. Upon completion of the cycling steps, a melt curve was created between 65°C and 95°C, increment 0.5°C for 5 s/step. Samples were run in duplicates in 384-well plates and results were analyzed using the Bio-Rad CFX maestro software. A standard curve using a pool of 22 or 24 dilution of all samples for all primers was assessed to determine the efficiency of the PCR reaction. mRNA expression was normalized to the mRNA content of three genes whose expression was not significantly different between groups (hydroxymethylbilane synthase, 60S acidic ribosomal protein, and TATA-binding protein, namely, Hmbs, Rplp0, and Tbp) and expressed as fold change compared with control mice using the ΔΔCt method.
Table 1.
Sequences of forward and reverse primers used for qPCR
| Name | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| Ldha | NM_010699 | GCAGTGGAAGGAGGTTCACAA | GAAGTGCTAGGACACGGGGA |
| Pdha1 | NM_008810 | ACACAGCATGAGTGACCCTG | CACCCATCCACCCACCTAAC |
| Pdk1 | NM_172665 | TCCTTAGAGGGCTACGGGAC | GCTTCCAGGCGGCTTTATTG |
| Cs | NM_026444 | AAGTTGGCAAAGACGTGTCA | CCGAGACACTCCAAACAGGAC |
| Hmbs | NM_013551 | TCTGCAAACGGGAAAACCCT | CCAGGACGATGGCACTGAAT |
| Rplp0 | NM_007475 | AGAAACTGCTGCCTCACATC | CATCACTCAGAATTTCAATGG |
| Tbp | NM_013684 | TGTATCTACCGTGAATCTTGGC | CCAGAACTGAAAATCAACGCAG |
Cs, citrate synthase; Hmbs, hydroxymethylbilane synthase; Ldha, Lactate dehydrogenase A; Pdha1, pyruvate dehydrogenase E1 alpha 1; Pdk1, pyruvate dehydrogenase kinase - isoenzyme 1; Rplp0, 60S acidic ribosomal protein; Tbp, TATA-binding protein.
Statistical Analyses and Data Presentation
For the analyses of body weight, GTT, and ITT, two-way ANOVAs for repeated measures were used within each group with time and IH as grouping variables. If significant effects or interactions appeared, a post hoc Fisher’s least-significant different (LSD) analysis was used to assess the effect of IH exposures at different time points of the experiment. All parameters obtained under baseline conditions after the 6-h fasting period and the areas under the curve for ITT and GTT were analyzed with two-way ANOVAs, using groups and IH as grouping variables. If significant effects or interactions appeared, a post hoc Fisher’s LSD analysis was used to assess significant effects. All values are reported as means ± SD or as boxes and whiskers showing the median, 25th and 75th percentiles, and minimum and maximum values. All analyses have been done with GraphPad Prism for macOS (v. 8.4.2).
RESULTS
IH Induces Sex-Specific Reductions of Body Weight, Fasting Glucose, and Insulin Levels
Levels of E2 were below the lower limit of detection of the assay kit (6 pg/mL) in the majority of samples from the intact males (7 low samples for 10 total samples), intact females (4/8 samples), and OVX-Veh females (7/9 samples), yielding insufficient data for meaningful comparisons. In OVX-E2 female mice, plasma E2 levels were respectively measured at 40.2 ± 31.9 and 19.9 ± 6.0 pg/mL in Nx and IH mice, without significant difference between groups (t test P value = 0.2).
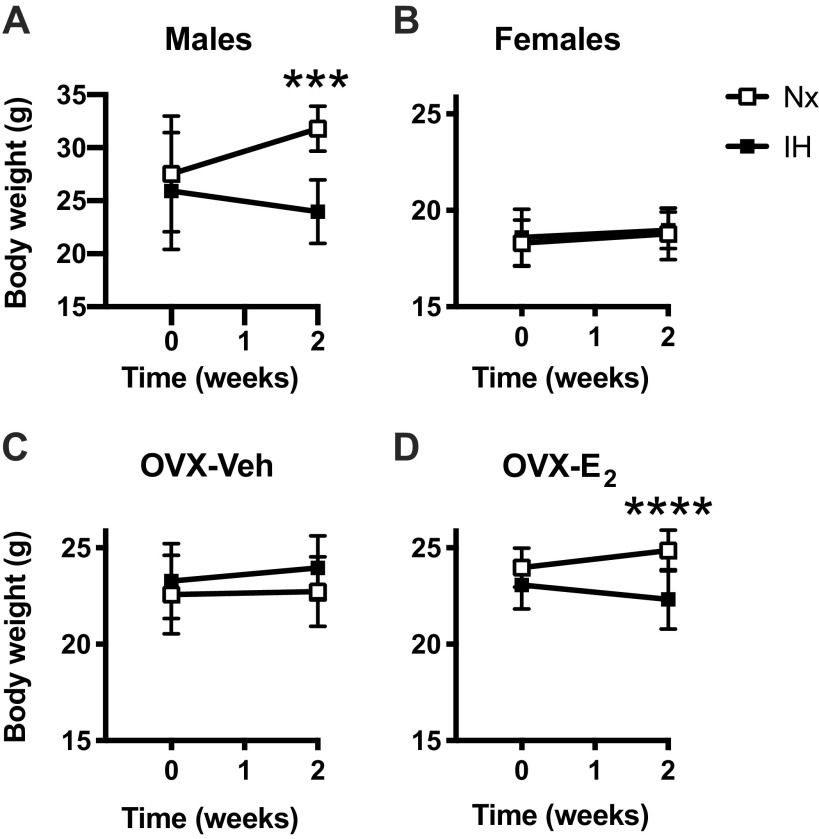
Male mice exposed to IH had a lower body weight (24.0 ± 3.0 g) than mice maintained in Nx (31.8 ± 2.1 g, Fig. 1A), this was not observed in intact or OVX-Veh females (Fig. 1, B and C), but a similar effect appeared in OVX-E2 mice. Fasting levels of glucose and insulin decreased by ∼39% and 64%, respectively, after IH exposures in male mice, and by 12% and 53% in OVX-Veh females (Table 2). In OVX-E2 mice, IH exposures slightly decreased glucose levels (−11%) and increased insulin levels (+ 86% - Table 2). It is noteworthy that glucose and insulin levels were lower in intact females than in males, glucose levels were increased in OVX-Veh, and both glucose and insulin levels were reduced by E2 supplementation (Table 2).
Figure 1.
Effects of exposures to normoxia (Nx) or intermittent hypoxia (IH) for 2 wk on body weight in male (A), female (B), ovariectomized-vehicle (OVX-Veh) (C), and ovariectomized- estradiol (OVX-E2) (D) mice. All values are means ± SD. ***P < 0.001 and ****P < 0.0001, IH vs. Nx.
Table 2.
Fasting blood levels of glucose and insulin, HOMA-IR, and HOMA-β in males, females, OVX-Veh and OVX-E2 female mice exposed to normoxia (NX) or intermittent hypoxia (IH)
| Males | Females | OVX-Veh | OVX-E2 | |
|---|---|---|---|---|
| Glucose, mM | ||||
| Nx | 10.8 ± 1.4 (n = 10) | 7.1 ± 0.7 (n = 9)°°°° | 8.3 ± 1.2 (n = 15)°° | 7.0 ± 0.7 (n = 10)°° |
| IH | 6.6 ± 1.2**** (n = 10) | 7.1 ± 0.8 (n = 11) | 7.3 ± 1.1 (n = 13)** | 6.0 ± 0.8 (n = 13)*/°°° |
| Insulin, ng/mL | ||||
| Nx | 2.1 ± 0.3 | 0.8 ± 0.6°°°° | 1.0 ± 0.4 | 0.6 ± 0.3° |
| IH | 0.8 ± 0.4**** | 0.6 ± 0.4 | 0.5 ± 0.3*** | 1.2 ± 0.4**/°°°° |
| HOMA-IR, I × G /22.5 | ||||
| Nx | 1.00 ± 0.21 | 0.25 ± 0.17°°°° | 0.38 ± 0.20 | 0.19 ± 0.09°° |
| IH | 0.24 ± 0.13**** | 0.19 ± 0.15 | 0.16 ± 0.10*** | 0.33 ± 0.15*/°° |
| HOMA-β, I/G | ||||
| Nx | 0.20 ± 0.05 | 0.11 ± 0.09°° | 0.12 ± 0.05 | 0.09 ± 0.06 |
| IH | 0.11 ± 0.06** | 0.08 ± 0.06 | 0.07 ± 0.03* | 0.19 ± 0.06***/°°°° |
Values are means ± SD. Number of animals within each group indicated as (n = x)
P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, IH vs. NX.
°P < 0.05, °°P < 0.01, °°°P < 0.001, and °°°°P < 0.0001 vs. precedent column (females vs. males, OVX-Veh vs. females, OVX-E2 vs. OVX-Veh).
The HOMA-IR and HOMA-β were reduced by IH exposures in male and OVX-Veh female mice (Table 2) indicating improved insulin sensitivity and reduced glucose-induced insulin release under fasting conditions. No such effects were reported in intact females, whereas in OVX-E2 females the HOMA-IR and HOMA-β were increased by IH exposures, suggesting decreased insulin sensitivity and enhanced insulin release under fasting conditions. Furthermore, HOMA-IR and HOMA β were lower in intact females than in intact males (Table 2).
IH Does Not Alter Insulin Tolerance in Male and Female Mice
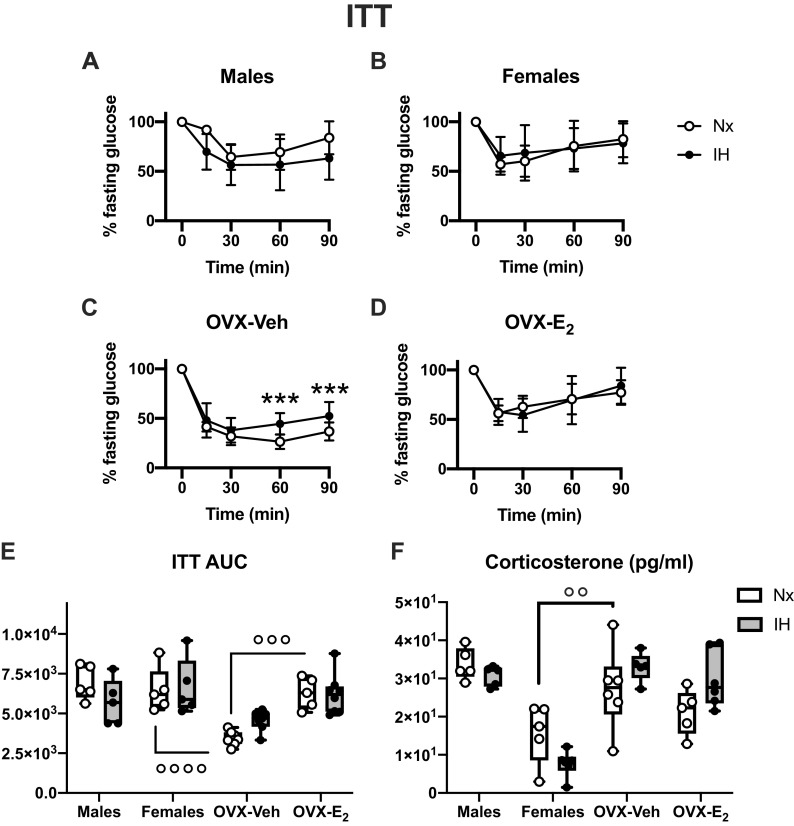
Because IH reduced fasting glucose levels in all groups except intact females, glucose concentrations during the ITT were normalized to fasting values. Intact males and females had similar blood glucose response following the intraperitoneal insulin injection (Fig. 2, A and B) and similar AUC (Fig. 2E), but insulin response was increased in OVX-Veh compared with intact females (Fig. 2, C and E), which was reversed by E2 supplementation (Fig. 2, D and E). IH had no effect on the ITT in intact male and female mice (Fig. 2, A and B). Contrastingly, in OVX-Veh mice, there was an interaction between IH and the glucose responses to insulin during the ITT (P = 0.017). OVX-Veh mice exposed to IH had higher glucose levels than Nx mice at 60 and 90 min after the insulin injection, suggesting some degree of insulin resistance. However, as IH only affected the late phase of the ITT, it likely represents a more efficient counterregulatory response to insulin-induced hypoglycemia than a physiologically relevant insulin resistance. In line with this, the plasma levels of corticosterone (involved in glucose control during metabolic stress) were higher in OVX-Veh than in intact female mice (Fig. 2F). In OVX-E2 mice exposed to IH, there was no significant interaction between IH and glucose responses during the ITT (P = 0.5, Fig. 2D), and the AUC for the ITT (Fig. 2E) was similar in IH and Nx OVX-E2 mice.
Figure 2.
Effects of exposures to normoxia (Nx) or intermittent hypoxia (IH) for 2 wk on insulin tolerance tests (ITT) in male (A), female (B), ovariectomized-vehicle (OVX-Veh) (C), and ovariectomized- estradiol (OVX-E2) (D) mice. ITT AUC (E): area under curve for the ITT (arbitrary units). Corticosterone (F): plasma corticosterone (pg/mL). Values for ITT are means ± SD. Boxes and whiskers (for AUC) show the median, 25th and 75th percentiles, minimum and maximum values, and individual data points. **P < 0.01, ***P < 0.001, IH vs. Nx. °°°P < 0.001 and °°°°P < 0.0001 between selected groups.
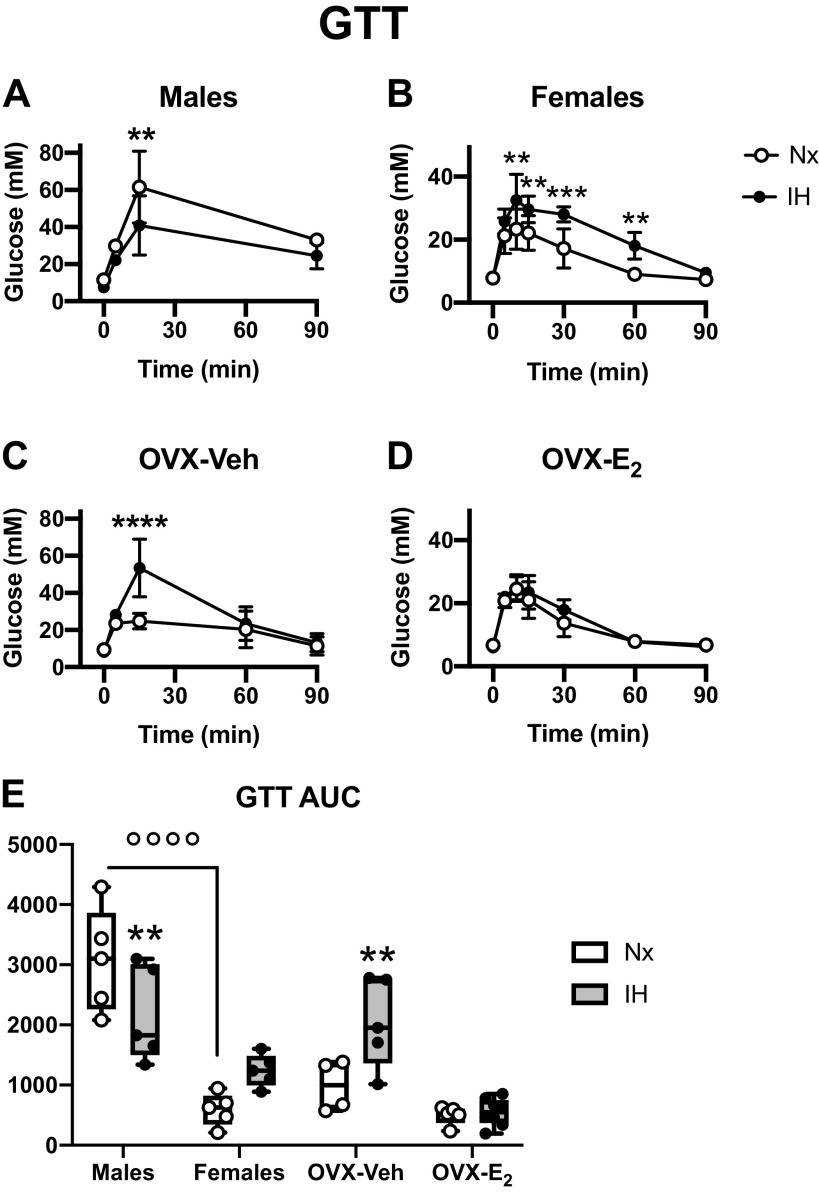
Figure 3.
Effects of exposures to normoxia (Nx) or intermittent hypoxia (IH) for 2 wk on glucose tolerance tests in male (A), female (B), ovariectomized-vehicle (OVX-Veh) (C), and ovariectomized- estradiol (OVX-E2) (D) mice. Glucose tolerance tests (GTT): blood glucose concentration measured after an ip glucose injection (2 g/kg – A–D). GTT AUC (E): area under curve for the GTT (arbitrary units). Values for GTT are means ± SD. Boxes and whiskers (for AUC) show the median, 25th and 75th percentiles, minimum and maximum values, and individual data points. **P < 0.01, ***P < 0.001, and ****P < 0.0001, IH vs. Nx. °°°°P < 0.0001 between selected groups.
IH Induces Sex-Specific Effects of Glucose Tolerance That Are Regulated by E2 in Females
Compared with intact males, intact females exposed to Nx reached lower glucose concentration (Fig. 3, A and B) and had lower AUC during the GTT (Fig. 3E), and there was no effect of ovariectomy or E2 supplementation in normoxia (Fig. 3, D and E). Glucose tolerance was improved in male mice exposed to IH, as evidenced by the lower level of blood glucose during the GTT and the lower AUC (Fig. 3, A and E). In females, IH exposures had an opposite effect, inducing a glucose intolerance, with blood glucose reaching higher values during the GTT (Fig. 3B), and a clear tendency for higher AUC (although the post hoc analysis P value was 0.058 for IH in female mice, Fig. 3E). This effect was more pronounced in OVX-Veh mice, IH exposures induced glucose intolerance as evidenced by the high glucose levels reached and elevated AUC (Fig. 3, C and E). However, this was successfully prevented by E2 supplementation (Fig. 3, D and E).
IH Induces Sex-Specific Profiles of Metabolic Genes Expression in the Liver That Are Regulated by E2 in Females
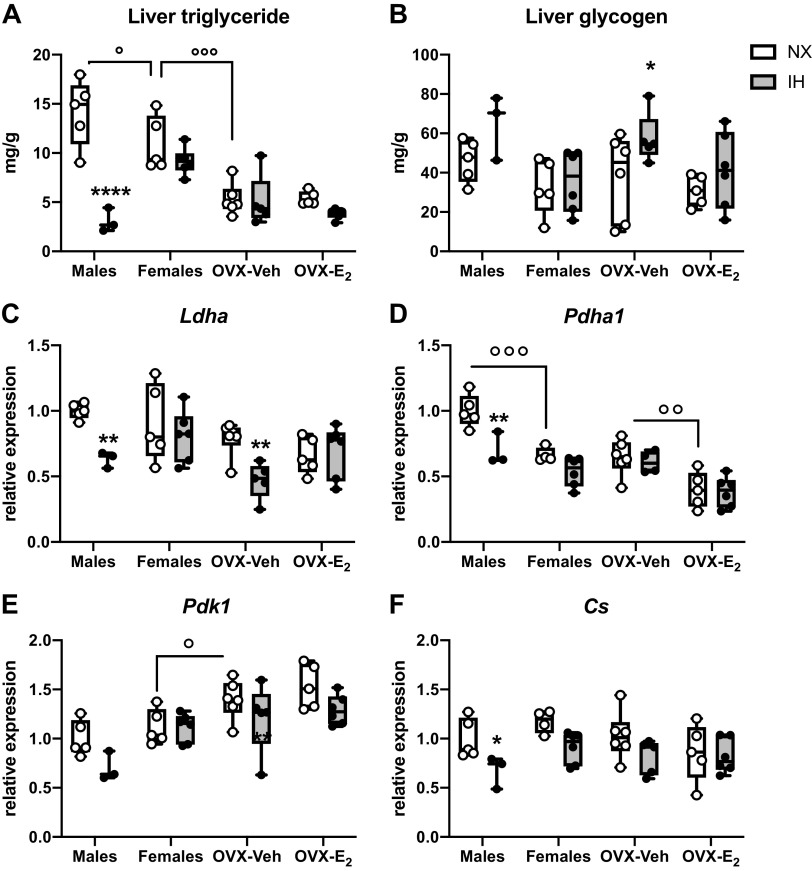
Triglyceride levels were lower in intact females than in males and was further reduced in OVX-Veh females (Fig. 4A). In male mice, IH drastically decreased the concentration of TG in the liver (−80%), but not in other groups (P value <0.0001 for interaction between IH and groups, Fig. 4A). There was a significant effect of IH (ANOVA, P value = 0.015) for the concentration of glycogen, but the post hoc analysis showed a significant effect only in OVX-Veh females (Fig. 4B). Interestingly, the expression levels of key genes involved in aerobic (Pdha1, Pdk1, and Cs, Fig. 4, D–F) and anaerobic (Ldha; Fig. 4C) glycolysis were lower in male mice exposed to IH, suggesting lower metabolic activity in the liver, in line with reduced circulating glucose. Similarly, in OVX-Veh female mice, the expression levels of Ldh was reduced by IH exposures, whereas there was no effect in intact females and OVX-E2 females.
Figure 4.
Effects of intermittent hypoxia (IH) exposures, sex, ovariectomized (OVX), and estradiol (E2) supplementation on liver functions and expression of key genes involved in aerobic and anaerobic metabolism. A: concentrations of triglyceride in the liver (mg/g tissue). B: concentrations of glycogen in the liver (mg/g tissue). D–F: relative expression level of mRNA coding lactate dehydrogenase A (Ldha), pyruvate dehydrogenase alpha 1 subunit (Pdha1), pyruvate dehydrogenase kinase 1 (Pdk1), citrate synthase [Cs – all values for gene expression levels normalized to males normoxia (Nx)]. Boxes and whiskers show the median, 25th and 75th percentiles, minimum and maximum values and individual data points. *P < 0.05, **P < 0.01, and ****P < 0.0001, IH vs. Nx. °P < 0.05, °°P < 0.01, and °°°P < 0.001 between selected groups. n = 3–6 mice/group.
DISCUSSION
To the best of our knowledge, our results show for the first time that an animal model of sleep apnea in mice induces sex-specific metabolic effects, and that some of these effects are mediated by E2 in females. The key findings of this study are that 1) IH exposures reduced fasting insulin and glucose levels and the expression of genes involved in aerobic and anaerobic metabolism in the liver in males and in OVX-Veh females. 2) IH improves glucose tolerance only in males. 3) In females, IH exposures decreased glucose tolerance, this effect was amplified in OVX-Veh females, and successfully reversed by E2 supplementation. 4) During IH exposures, E2 supplementation appears to improve pancreatic β-cell functions.
An intriguing observation from this study is that for some aspects of metabolic regulations (fasting glucose and insulin levels, and expression pattern of hepatic genes), the OVX-Veh female demonstrated “male-like” responses to IH, whereas for other aspects (ITT, GTT), they demonstrated responses that were not observed in males. The origin of this divergent pattern of responses between males and OVX-Veh females remains thus unknown and could imply some effects of sex-hormones in males.
The apparent protective role of E2 during IH exposures in female mice may, at least partially, explain the sex-specific comorbidities associated with sleep apnea in humans and the differences between pre- and postmenopausal women with sleep apnea regarding the development of type 2 diabetes (2, 3, 6). Clearly, the underlying mechanisms need further investigation.
Contrasting with several other studies (7, 8, 11, 19, 20), we did not report insulin resistance in male mice exposed to IH. On the contrary, our model of IH exposures apparently improve insulin sensitivity in males, at least under the fasting condition as shown by decreased HOMA-IR index. Experimental protocols may be one of the key differences between previous studies and our present findings. Although we used a low hypoxia level (6% O2), a frequency of 10 cycles/h is relatively modest compared with most other studies that used higher IH frequencies ranging from 20 to 60 cycles/h, simulating severe apneic cases (7, 8, 11, 19, 20). Under these conditions, it has been established that IH exposures induce inflammation of the visceral adipose tissue, and long-lasting impairments of metabolic activity in adipocytes, contributing to the establishment of insulin resistance (7, 8, 21, 22). A potential hypothesis is that only high frequencies of IH exposures induce insulin resistance in mice, but the fact that lean mice exposed to IH at frequencies of 12 or 60 cycles/h (nadir 5% O2) had similar insulin resistance assessed by fasting glucose and insulin levels disproves this hypothesis (23). It is noteworthy that a frequency of arterial oxygen desaturation of 10 events/h is a moderate clinical degree of apnea frequency. Therefore, our results indicate that sex-specific and E2-dependent metabolic consequences could occur and contribute to long-term metabolic health alterations in these patients.
It is well established that lean female mice are generally more sensitive to insulin and more tolerant to glucose than their respective male counterparts (24, 25). In C57BL/6 mice, this sex-specific metabolic variation has been attributed to higher insulin sensitivity in adipose tissue and liver (24). Our results are consistent with this, showing higher insulin sensitivity in females than in males, as suggested by the lower HOMA-IR under fasting conditions (cf Table 2). Interestingly, an effect of IH on the glucose levels during the ITT clearly appears during the secondary phase of the tolerance test (from 30 min after the insulin injection) in OVX-Veh females. This could be linked to the counterregulatory responses to hypoglycemia increasing compensatory glycogenolysis or gluconeogenesis in the liver. In line with this, the levels of corticosterone—one of the key hormones inducing gluconeogenesis in response to metabolic stress (26)—were increased by OVX in female mice, and it is, therefore, possible that this accounts for some of the effects induced by IH during the ITT in this group.
As previously reported by others (11, 19, 20), IH improved glucose tolerance in male mice (Fig. 3). This effect has been attributed to an increase glucose uptake by skeletal muscles, mediated by an increased activity of the AMP-activated protein kinase (AMPK) metabolic sensor (11). Our results show that IH had opposite effects on glucose tolerance in male versus females, therefore suggesting that the underlying mechanisms are modulated by sex steroids. Furthermore, IH exposures induced an important glucose intolerance in OVX females that was successfully reversed by E2 supplementation. However, OVX mice did not exhibit a “male-like” phenotype implying that ovarian hormones are not sufficient to explain the sex-specific GTT response to IH. This simple observation opens intriguing research questions concerning the role of gonadal hormones in male mice during IH exposures. Interestingly, previous studies have reported that E2 activates AMPK through a nongenomic effect of the ERα receptor, which is able to directly bind the catalytic α subunit of AMPK (27). If such effects occur in our model, and lead to enhanced glucose uptake by skeletal muscles, this could contribute to reduce the glucose intolerance in intact and OVX-E2 mice compared with OVX-Veh. Although we cannot further speculate on the precise mechanisms underlying the sex-specific effects of IH on glucose tolerance in mice, or on the interactions with endogenous sex-hormones in males and females, this line of research clearly deserves further studies in IH models.
HOMA-β, the ratio of insulin to glucose levels in fasting conditions, is a surrogate marker of pancreatic β cells response to glucose (16, 28). In males and OVX-Veh females, IH exposures decrease HOMA-β, but this is not observed in intact females, suggesting a sex-specific and hormone-dependent effect of IH on pancreatic β cell functions. This result is consistent with previous studies showing that in male mice IH exposures for 14 days increase pancreatic oxidative stress and reduce insulin release induced by glucose injection (28). Interestingly, this is further enhanced after a normoxic recovery period of 7 days following the IH exposures, suggesting long-lasting effects (28). It has also been reported that IH induces mitochondrial oxidative stress in pancreatic β cells, decreases the insulin content of islets, and impairs glucose-induced insulin release (29). Furthermore, in a mouse model of type 2 diabetes, IH induces apoptosis in pancreatic β cells (9).
In OVX-E2 females, IH increased HOMA-β, suggesting improved pancreatic β cell functions. The effects of E2 on β cells are related to the presence of ERα and Erβ that are able to prevent pancreatic dysfunction in most rodent models of diabetes (30). Activation of Erα enhances insulin synthesis, reduces oxidative stress, and prevents apoptosis of pancreatic β cells induced by streptozotocin (30–32), whereas Erβ increases insulin secretion induced by glucose (33). Therefore, our results strongly suggest a similar effect of E2 against oxidative stress in pancreatic β cells and impaired glucose-induced insulin release. It is also tempting to hypothesize that the effects of E2 are due to a protective effect of Erα and Erβ against mitochondrial oxidative stress induced by IH, as previously demonstrated in the brain cortex of adult female rats (34).
The metabolic profile in the liver and the expression pattern for the genes involved in aerobic and anaerobic glucose metabolism are consistent with the systemic changes of body weight and glucose homeostasis. IH reduces the concentration of TG in the liver in males, with a clear tendency for a similar effect in OVX-E2 females, concomitant with a reduced body weight. In males and OVX-Veh females, the lower expression of genes involved in aerobic and anaerobic metabolism induced by IH likely indicates a reduced metabolic capacity that would suit the lower fasting blood glucose concentrations. This implies, at least for the liver, that under our conditions of IH exposures, male and OVX-Veh female mice display an adequate metabolic flexibility, defined as the capacity for an organism to adapt fuel oxidation to fuel availability (35). It is noteworthy that such flexibility helps protect against tissue and whole body metabolic dysfunctions, and therefore, the hepatic response to IH in male and OVX-Veh female mice appears adequate.
Finally, it is worth mentioning that in male (36) and OVX-Veh female (37) rats, exposure to IH reduces whole body energetic expenditure as measured by O2 consumption and CO2 production rates under normoxia, and E2 supplementation reversed this phenotype in the OVX females (37). Because liver metabolic activity accounts for ≈50% of the resting O2 consumption in mice (38), it is tempting to speculate that low metabolic activity of the liver contributes to the reduced whole body metabolic rates induced by IH exposures in rodents.
One of the limitations of this study is that the estrous cycle of intact females has not been evaluated. However, variability of the reported data in the group of intact females was not higher than in other groups, likely indicating that this is not a confounding factor.
Overall, these results are relevant to better understand sex-specific and hormone-dependent metabolic risk factors associated with sleep apnea (3–6). This adds to previous work that we performed at the whole body level, or ex vivo, showing the role of E2 (34, 37, 39) or progesterone (40), during exposures to IH in female rats. Altogether, these results highlight that the interactions between sex-hormones and IH alter several physiological and pathophysiological responses to IH. These are most certainly the emerging tip of a vast “knowledge iceberg” that still remains to be fully and coherently unraveled by further research efforts.
GRANTS
This study was funded by the Canadian Institutes of Health Research (Funding Reference No: 162232) and Fondation de l’Institut Universitaire de Cardiologie et Pneumologie de Québec/fonds sur les maladies respiratoires J. -D. Bégin – P. H. Lavoie de l’Université Laval.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L. and V.J. conceived and designed research; F.M., A.J.-L., and M.M. performed experiments; F.M., A.J.-L., G.G.-C., and V.J. analyzed data; F.M., A.J.-L., G.G.-C., M.L., A.M., A.B., and V.J. interpreted results of experiments; G.G.-C. and V.J. prepared figures; A.B. and V.J. drafted manuscript; F.M., A.J.-L., M.L., A.M., A.B., and V.J. edited and revised manuscript; F.M., A.J.-L., G.G.-C., M.M., M.L., A.M., A.B., and V.J. approved final version of manuscript.
ACKNOWLEDGMENTS
A.J.-L., G.G.-C., A.B., and V.J. are members of the Physiological Breathing Disorders Axis of the Quebec Respiratory Health Network. The authors acknowledge the contribution of Vanessa Houde and Yves Gélinas for helpful support and advices.
REFERENCES
- 1.Block A, Boysen P, Wynne J, Hunt L. Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. N Engl J Med 300: 513–517, 1979. doi: 10.1056/NEJM197903083001001. [DOI] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3: 310–318, 2015. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath 22: 241–249, 2018. doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 4.Cano-Pumarega I, Barbé F, Esteban A, Martínez-Alonso M, Egea C, Durán-Cantolla J; Spanish Sleep Network . Sleep apnea and hypertension: are there gender differences? The Vitoria sleep cohort. Chest 152: 742–750, 2017. doi: 10.1016/j.chest.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Heinzer R, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Waeber G, Preisig M, Vollenweider P, Haba-Rubio J. Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med 51: 29–36, 2018. doi: 10.1016/j.sleep.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J 47: 1162–1169, 2016. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 7.Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, Mutlu GM, Akbarpour M, Gozal D. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: effect of normoxic recovery. Sleep 40, 2017. doi: 10.1093/sleep/zsw074. [DOI] [PubMed] [Google Scholar]
- 8.Murphy AM, Thomas A, Crinion SJ, Kent BD, Tambuwala MM, Fabre A, Pepin JL, Roche HM, Arnaud C, Ryan S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J 49: 1601731, 2017. doi: 10.1183/13993003.01731-2016. [DOI] [PubMed] [Google Scholar]
- 9.Sherwani SI, Aldana C, Usmani S, Adin C, Kotha S, Khan M, Eubank T, Scherer PE, Parinandi N, Magalang UJ. Intermittent hypoxia exacerbates pancreatic beta-cell dysfunction in a mouse model of diabetes mellitus. Sleep 36: 1849–1858, 2013. doi: 10.5665/sleep.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olea E, Agapito MT, Gallego-Martin T, Rocher A, Gomez-Nino A, Obeso A, Gonzalez C, Yubero S. Intermittent hypoxia and diet-induced obesity: effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J Appl Physiol 117: 706–719, 2014. doi: 10.1152/japplphysiol.00454.2014. [DOI] [PubMed] [Google Scholar]
- 11.Thomas A, Belaidi E, Moulin S, Horman S, van der Zon GC, Viollet B, Levy P, Bertrand L, Pepin JL, Godin-Ribuot D, Guigas B. Chronic intermittent hypoxia impairs insulin sensitivity but improves whole-body glucose tolerance by activating skeletal muscle AMPK. Diabetes 66: 2942–2951, 2017. doi: 10.2337/db17-0186. [DOI] [PubMed] [Google Scholar]
- 12.Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2: 227–242, 2013. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ Res 89: 534–539, 2001. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 14.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270, 2008. doi: 10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Caron A, Mouchiroud M, Gautier N, Labbe SM, Villot R, Turcotte L, Secco B, Lamoureux G, Shum M, Gelinas Y, Marette A, Richard D, Sabatini DM, Laplante M. Loss of hepatic DEPTOR alters the metabolic transition to fasting. Mol Metab 6: 447–458, 2017. doi: 10.1016/j.molmet.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo S, Russell JC, Taylor AW. Determination of glycogen in small tissue samples. J Appl Physiol 28: 234–236, 1970. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 19.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol 303: R700–R709, 2012. doi: 10.1152/ajpregu.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carreras A, Zhang SX, Almendros I, Wang Y, Peris E, Qiao Z, Gozal D. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology 156: 437–443, 2015. doi: 10.1210/en.2014-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, Khalyfa AA, Andrade J, Khalyfa A. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. Am J Respir Cell Mol Biol 56: 477–487, 2017. doi: 10.1165/rcmb.2016-0243OC. [DOI] [PubMed] [Google Scholar]
- 23.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol (1985) 111: 881–890, 2011. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goren HJ, Kulkarni RN, Kahn CR. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology 145: 3307–3323, 2004. doi: 10.1210/en.2003-1400. [DOI] [PubMed] [Google Scholar]
- 25.Parks BW, Sallam T, Mehrabian M, Psychogios N, Hui ST, Norheim F, Castellani LW, Rau CD, Pan C, Phun J, Zhou Z, Yang WP, Neuhaus I, Gargalovic PS, Kirchgessner TG, Graham M, Lee R, Tontonoz P, Gerszten RE, Hevener AL, Lusis AJ. Genetic architecture of insulin resistance in the mouse. Cell Metab 21: 334–347, 2015. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimeno B, Hau M, Verhulst S. Corticosterone levels reflect variation in metabolic rate, independent of 'stress'. Sci Rep 8: 13020, 2018. doi: 10.1038/s41598-018-31258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipovka Y, Chen H, Vagner J, Price TJ, Tsao TS, Konhilas JP. Oestrogen receptors interact with the alpha-catalytic subunit of AMP-activated protein kinase. Biosci Rep 35: e00264, 2015. doi: 10.1042/BSR20150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polak J, Shimoda LA, Drager LF, Undem C, McHugh H, Polotsky VY, Punjabi NM. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep 36: 1483–1490; 1490A–1490B, 2013. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic beta-cell function by chronic intermittent hypoxia. Exp Physiol 98: 1376–1385, 2013. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol 8: 342–351, 2012. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 3: e2069, 2008. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103: 9232–9237, 2006. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, Kuhn M, Gustafsson JA, Nadal A. Rapid regulation of K(ATP) channel activity by 17{beta}-estradiol in pancreatic {beta}-cells involves the estrogen receptor {beta} and the atrial natriuretic peptide receptor. Mol Endocrinol 23: 1973–1982, 2009. doi: 10.1210/me.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laouafa S, Roussel D, Marcouiller F, Soliz J, Gozal D, Bairam A, Joseph V. Roles of oestradiol receptor alpha and beta against hypertension and brain mitochondrial dysfunction under intermittent hypoxia in female rats. Acta Physiol 226: e13255, 2019. doi: 10.1111/apha.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–E1017, 2008. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan BJ, Adrian R, Wang ZY, Bates ML, Dopp JM. Chronic intermittent hypoxia alters ventilatory and metabolic responses to acute hypoxia in rats. J Appl Physiol (1985) 120: 1186–1195, 2016. doi: 10.1152/japplphysiol.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laouafa S, Ribon-Demars A, Marcouiller F, Roussel D, Bairam A, Pialoux V, Joseph V. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep 40, 2017. doi: 10.1093/sleep/zsx104. [DOI] [PubMed] [Google Scholar]
- 38.Kummitha CM, Kalhan SC, Saidel GM, Lai N. Relating tissue/organ energy expenditure to metabolic fluxes in mouse and human: experimental data integrated with mathematical modeling. Physiol Rep 2: e12159, 2014. doi: 10.14814/phy2.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribon-Demars A, Pialoux V, Boreau A, Marcouiller F, Lariviere R, Bairam A, Joseph V. Protective roles of estradiol against vascular oxidative stress in ovariectomized female rats exposed to normoxia or intermittent hypoxia. Acta Physiol (Oxf) 225: e13159, 2019. doi: 10.1111/apha.13159. [DOI] [PubMed] [Google Scholar]
- 40.Joseph V, Laouafa S, Marcouiller F, Roussel D, Pialoux V, Bairam A. Progesterone decreases apnoea and reduces oxidative stress induced by chronic intermittent hypoxia in ovariectomized female rats. Exp Physiol 105: 1025–1034, 2020. doi: 10.1113/EP088430. [DOI] [PubMed] [Google Scholar]