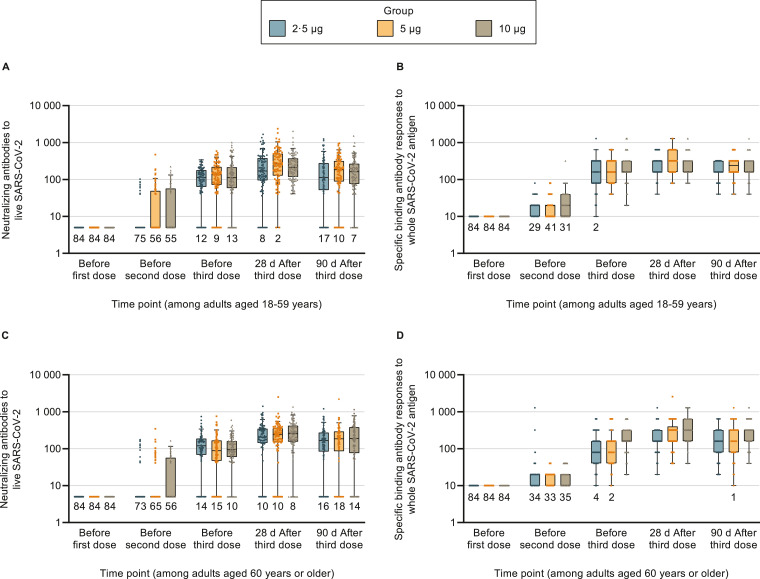
Fig. 2.
Antibody responses of participants receiving 3 doses of vaccines in the phase 1 and 2 trials at different time points
Data from the phase 1 and 2 trials were combined. Placebo groups are not shown since no measurable antibody responses could be detected in these groups at any time. There were 84 participants in each group. Among participants aged ≥60 years in the medium-dose group, 1, 1, and 2 participants did not provide blood samples before the second dose, before the third dose, and on days 28 and 90 after the whole-course vaccination. Among participants aged ≥60 years in the high-dose group, 1, 1, and 3 participants did not provide blood samples before the second dose, before the third dose, and on days 28 and 90 after the whole-course vaccination. The dots represent individual participant values. The boxes and horizontal bars denote interquartile range (IQR) and median value. The whisker's upper endpoint equaled to 75th percentile + 1•5 × IQR, and the whisker's lower endpoint equaled to 25th percentile - 1•5 × IQR. The numbers below the boxes indicate the number of participants with values below the detection limit. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.