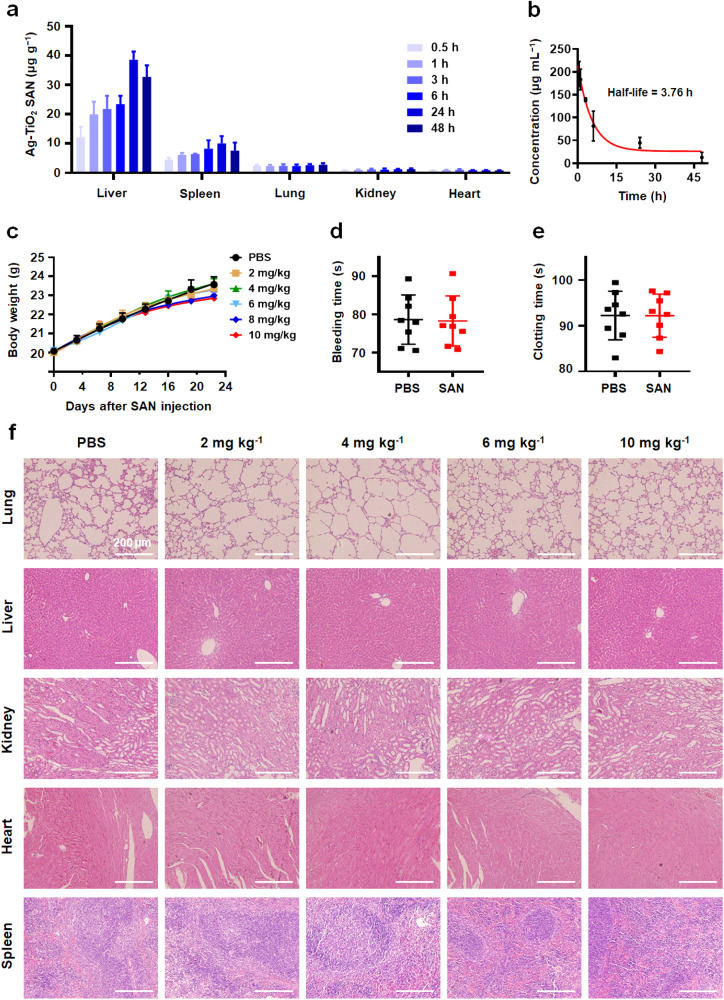
Fig. 5.
Pharmacokinetics and biocompatibility of Ag-TiO2 SAN. (a) The distribution of Ag-TiO2 SAN, including liver, spleen, lung, kidney, and heart, 0.5–48 h after intravenous administration (n = 4). (b) The blood circulation curve of intravenously injected Ag-TiO2 SAN (n = 4). The half-life was calculated to be 3.76 h. (c) Body weight curves of mice that were intravenously injected with PBS or 2–10 mg kg−1 Ag-TiO2 SAN (n = 10). (d-e) The statistics of bleeding and clotting times of mice that were intravenously injected with PBS or 10 mg kg−1 Ag-TiO2 SAN (n = 8). (f) HE staining of main organs of mice that were intravenously injected with PBS or 2–10 mg kg−1 Ag-TiO2 SAN for 24 days (n = 3).