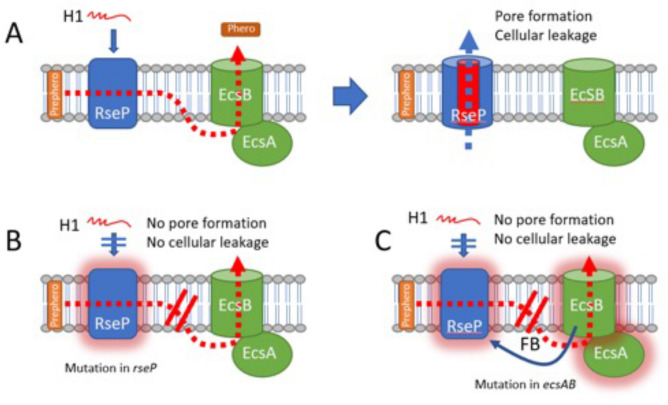
Figure 5.
Proposed model for the EcsAB mediated H1-resistance in S.haemolyticus. (A) RseP is a inner-membrane protease working together the ABC-transporter EcsAB to cleave the hormone prepeptide (Prephero) and export the mature hormone peptide (Phero) to the external milieu. RseP is acting also as the receptor for the bacteriocin H1 which forms pores and causes lethal cellular leakage across the membrane. (B) When rseP is mutated resulting in a non-functional receptor (RseP with glow), cells become resistant to H1. (C) When the ecs system is mutated, the malfunctional ABC transporter can no longer export the hormone peptide. This jammed situation of hormone prepeptide/peptide within the membrane results in a feedback (FB) loop somehow causing RseP inactivity. An inactive RseP (RseP with glow) loses its function as a bacteriocin receptor, and cells therefore become resistant to H1.